Analysis of effect of preoperative renal insufficiency on clinical prognosis of heart transplant recipients
-
摘要:
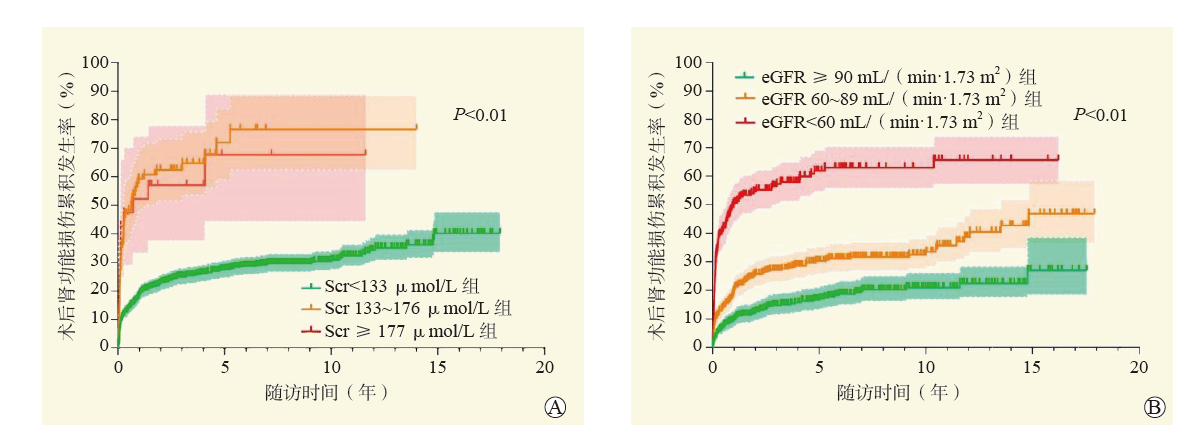
目的 评估心脏移植术前合并肾功能不全对围手术期死亡和并发症发生及长期生存的影响,并比较术前血清肌酐(Scr)和估测肾小球滤过率(eGFR)在术前风险评估中的差异。 方法 回顾性分析1 095例心脏移植受者的临床资料,根据术前Scr分为Scr < 133 μmol/L组(980例)、Scr 133~176 μmol/L组(83例)和Scr≥177 μmol/L组(32例);根据术前eGFR分为eGFR≥90 mL/(min·1.73 m2)组(436例)、eGFR 60~89 mL/(min·1.73 m2)组(418例)和eGFR < 60 mL/(min·1.73 m2)组(241例)。分析不同分组受者术后肾功能的转归情况及围手术期和远期结局。评价eGFR和Scr对心脏移植术后肾功能损伤和远期生存的影响。 结果 随着术前Scr升高,受者术后使用连续性肾脏替代治疗(CRRT)的比例增加,术后机械循环辅助的比例增加,术后并发症发生率增加,机械通气时间和重症监护室(ICU)入住时间延长,院内病死率增加,3组间差异均有统计学意义(均为P < 0.05)。随着术前eGFR的下降,受者术后使用CRRT辅助的比例增加,术后使用主动脉内球囊反搏(IABP)的比例增加,机械通气时间和ICU入住时间延长,院内病死率增加,3组间差异均有统计学意义(均为P < 0.05)。Scr≥177 μmol/L是受者术后死亡的独立危险因素[校正风险比(HR)3.64,95%可信区间(CI)1.89~6.99,P < 0.01]。以Scr及以eGFR为指标的分组中,3组间的术后肾功能损伤累积发生率和远期生存率差异均有统计学意义(均为P < 0.05)。术前Scr < 133 μmol/L的受者中,术后远期肾功能损伤随时间的累积发生率随术前eGFR降低而升高(P < 0.01),而不同eGFR分层的患者术后远期生存率差异无统计学意义(P > 0.05)。 结论 心脏移植术前合并肾功能不全与围手术期和远期预后不良相关。心脏移植术前Scr和eGFR均是术后肾功能损伤发生的独立危险因素。Scr对于术前肾功能评估的灵敏度较低,但预测围手术期死亡风险的准确性更高。eGFR是术前评估肾功能更为敏感的指标,可以早期发现肾功能异常,早期采取有效措施从而减少对预后的不良影响。 -
关键词:
- 终末期心力衰竭 /
- 心脏移植 /
- 肾功能不全 /
- 血清肌酐 /
- 估计肾小球滤过率 /
- 连续性肾脏替代治疗(CRRT) /
- 体外膜肺氧合(ECMO) /
- 主动脉内球囊反搏(IABP)
Abstract:Objective To evaluate the effect of renal insufficiency before heart transplantation on perioperative death, complications and long-term survival, and to compare the differences between preoperative serum creatinine (Scr) and estimated glomerular filtration rate (eGFR) in preoperative risk assessment. Methods Clinical data of 1 095 heart transplant recipients were retrospectively analyzed. According to preoperative Scr level, all recipients were divided into the Scr < 133 μmol/L(n=980), Scr 133-176 μmol/L (n=83) and Scr≥177 μmol/L groups (n=32). According to preoperative eGFR, all recipients were divided into eGFR≥90 mL/(min·1.73m2) (n=436), eGFR 60-89 mL/(min·1.73m2) (n=418) and eGFR < 60 mL/(min·1.73m2) groups (n=241). Clinical prognosis of postoperative renal function, perioperative and long-term outcomes of recipients were compared among different groups. The effect of eGFR and Scr level on renal function injury and long-term survival after heart transplantation was assessed. Results With the increase of preoperative Scr level, the proportion of recipients undergoing postoperative continuous renal replacement therapy (CRRT) was increased, the proportion of recipients receiving postoperative mechanical circulatory support was elevated, the incidence of postoperative complications was increased, the duration of mechanical ventilation and intensive care unit(ICU) stay was prolonged, and the in-hospital fatality was increased. The differences among three groups were statistically significant (all P < 0.05). With the decrease of preoperative eGFR, the proportion of recipients receiving postoperative CRRT was increased, the proportion of recipients using postoperative intra-aortic balloon pump (IABP) was elevated, the duration of mechanical ventilation and ICU stay was prolonged, and the in-hospital fatality was increased. The differences among three groups were statistically significant (all P < 0.05). Scr≥177 μmol/L was an independent risk factor for postoperative death [adjusted hazard ratio (HR) 3.64, 95% confidence interval (CI) 1.89-6.99, P < 0.01]. Among different groups classified by Scr and eGFR, the cumulative incidence rate of postoperative renal function injury and long-term survival rate were statistically significant among three groups (all P < 0.05). In patients with preoperative Scr < 133 μmol/L, the cumulative incidence rate of postoperative long-term renal function injury was significantly increased with the decrease of preoperative eGFR (P < 0.01). There was no significant difference in postoperative long-term survival rate among patients stratified by different eGFR (P > 0.05). Conclusions Renal insufficiency before heart transplantation is associated with poor perioperative and long-term prognosis. Preoperative Scr and eGFR are the independent risk factors for postoperative renal function injury. Scr yields low sensitivity in the assessment of preoperative renal function, whereas it has high accuracy in predicting perioperative death risk. And eGFR is a more sensitive parameter to evaluate preoperative renal function, which may identify early-stage renal functional abnormality and take effective measures during early stage to reduce adverse effect on prognosis. -
图 3 术前Scr < 133 μmol/L的不同eGFR分层的受者术后肾功能损伤累积发生率和生存率比较
注:A图为术前Scr < 133μmol/L的不同eGFR分层的受者术后肾功能损伤累积发生曲线;B图为术前Scr < 133μmol/L的不同eGFR分层的受者术后远期生存曲线。
Figure 3. Comparison of cumulative incidence of postoperative renal function injury and survival rate of recipients with different eGFR stratification with Scr < 133 μmol/L before surgery
表 1 不同分组受者的术后资料比较
Table 1. Comparison of postoperative data of recipients among different groups
指标 Scr(μmol/L) eGFR[mL/(min·1.73 m2)] Scr < 133(n=980) Scr 133~176(n=83) Scr≥177(n=32) P值 eGFR≥90(n=436) eGFR 60~89(n=418) eGFR < 60(n=241) P值 术后CRRT辅助[n(%)] 39(4.0) 10(12.0)a 14(43.8)a, b < 0.01 14(3.2) 19(4.5) 30(12.4)c, d < 0.01 术后机械循环辅助[n(%)] ECMO辅助 68(6.9) 6(7.2) 8(25.0)a, b < 0.01 35(8.0) 28(6.7) 19(7.9) 0.74 IABP辅助 146(14.9) 17(20.5) 13(40.6)a < 0.01 59(13.5) 65(15.6) 52(21.6)c 0.02 术后并发症[n(%)] 气管切开 36(3.7) 6(7.2) 7(21.9)a < 0.01 18(4.1) 19(4.5) 12(5.0) 0.87 二次开胸 54(5.5) 5(6.0) 6(18.8)a < 0.01 26(6.0) 24(5.7) 15(6.2) 0.97 感染 126(12.9) 11(13.3) 11(34.4)a, b < 0.01 59(13.5) 57(13.6) 32(13.3) 0.99 机械通气时间[M(P25, P75),h] 22(17,39) 26(19,41)a 64(27,194)a, b < 0.01 21(17,37) 23(18,40) 27(19,46)c < 0.01 ICU入住时间[M(P25, P75),d] 4.4(2.8,6.4) 4.8(3.6,6.8)a 8.9(5.6,17.5)a, b < 0.01 3.8(2.7,5.8) 4.6(3.0,6.7)c 5.2(3.7,7.7)c, d < 0.01 术后住院时间[M(P25, P75),d] 19(15,25) 19(16,28) 22(13,37) 0.49 18(15,25) 20(15,26) 19(15,27) 0.29 院内病死率[n(%)] 35(3.6) 5(6.0) 11(34.4)a, b < 0.01 10(2.3) 19(4.5) 22(9.1)c, d < 0.01 出院时肾功能状态[n(%)] < 0.01 < 0.01 肾功能储备代偿期 893(91.1) 64(77.1) 19(59.4)a 363(83.3) 186(44.5)c 44(18.3)c, d 肾功能不全期 40(4.1) 9(10.8)a 1(3.1) 54(12.4) 152(36.4)c 111(46.1)c 肾衰竭期 12(1.2) 5(6.0)a 1(3.1)a 9(2.1) 61(14.6)c 64(26.6)c, d 注:与Scr < 133 μmol/L组比较,aP < 0.05/3;与Scr 133~176 μmol/L组比较,bP < 0.05/3;与eGFR≥90 mL/(min·1.73 m2)组比较,cP < 0.05/3;与eGFR 60~89 mL/(min·1.73 m2)组比较,dP < 0.05/3。 -
[1] 郑珊珊, 刘盛, 唐汉韡, 等. 危重状态病人心脏移植的早期结果: 阜外医院单中心经验[J]. 器官移植, 2021, 12(4): 450-457. DOI: 10.3969/j.issn.1674-7445.2021.04.012.ZHENG SS, LIU S, TANG HW, et al. Early outcomes of heart transplantation in critical patients: single center experience of Fuwai Hospital[J]. Organ Transplant, 2021, 12(4): 450-457. DOI: 10.3969/j.issn.1674-7445.2021.04.012. [2] LALA A, ROWLAND JC, FERKET BS, et al. Strategies of wait-listing for heart transplant vs durable mechanical circulatory support alone for patients with advanced heart failure[J]. JAMA Cardiol, 2020, 5(6): 652-659. DOI: 10.1001/jamacardio.2020.0631. [3] SUAREZ-PIERRE A, IGUIDBASHIAN J, STUART C, et al. Appraisal of donation after circulatory death: how far could we expand the heart donor pool?[J]. Ann Thorac Surg, 2022, 114(3): 676-682. DOI: 10.1016/j.athoracsur.2022.01.042. [4] BAKHTIYAR SS, GODFREY EL, AHMED S, et al. Survival on the heart transplant waiting list[J]. JAMA Cardiol, 2020, 5(11): 1227-1235. DOI: 10.1001/jamacardio.2020.2795. [5] KUMAR A, HOWARD A, THOMAS CP. Estimated glomerular filtration rate at transplant listing and other predictors of post-heart transplant mortality and the development of ESRD[J]. Transplantation, 2020, 104(11): 2444-2452. DOI: 10.1097/TP.0000000000003159. [6] BARUA S, YANG T, CONTE S, et al. Value of renal histology in predicting cardiorenal outcomes in heart transplant-listed patients[J]. Transplant Direct, 2022, 9(1): e1424. DOI: 10.1097/TXD.0000000000001424. [7] CHEN JW, CHOU NK, WANG CH, et al. Impact of pretransplant renal replacement therapy on clinical outcome after isolated heart transplantation[J]. Transpl Int, 2022, 35: 10185. DOI: 10.3389/ti.2022.10185. [8] KIM D, CHOI JO, CHO YH, et al. Impact of preoperative renal replacement therapy on the clinical outcome of heart transplant patients[J]. Sci Rep, 2021, 11(1): 13398. DOI: 10.1038/s41598-021-92800-0. [9] KUMAR A, BONNELL LN, THOMAS CP. Severely reduced kidney function assessed by a single eGFR determination at the time of an isolated heart transplant does not predict inevitable posttransplant ESKD[J]. Transplantation, 2023, 107(4): 981-987. DOI: 10.1097/TP.0000000000004350. [10] HONG KN, IRIBARNE A, WORKU B, et al. Who is the high-risk recipient? predicting mortality after heart transplant using pretransplant donor and recipient risk factors[J]. Ann Thorac Surg, 2011, 92(2): 520-527. DOI: 10.1016/j.athoracsur.2011.02.086. [11] KILIC A, WEISS ES, ARNAOUTAKIS GJ, et al. Identifying recipients at high risk for graft failure after heart retransplantation[J]. Ann Thorac Surg, 2012, 93(3): 712-716. DOI: 10.1016/j.athoracsur.2011.10.065. [12] LUND LH, EDWARDS LB, KUCHERYAVAYA AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult heart transplantation report--2015; focus theme: early graft failure[J]. J Heart Lung Transplant, 2015, 34(10): 1244-1254. DOI: 10.1016/j.healun.2015.08.003. [13] ROVIN BH, ADLER SG, BARRATT J, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases[J]. Kidney Int, 2021, 100(4): 753-779. DOI: 10.1016/j.kint.2021.05.015. [14] LEVEY AS, STEVENS LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions[J]. Am J Kidney Dis, 2010, 55(4): 622-627. DOI: 10.1053/j.ajkd.2010.02.337. [15] SCICCHITANO P, IACOVIELLO M, PASSANTINO A, et al. The prognostic impact of estimated creatinine clearance by bioelectrical impedance analysis in heart failure: comparison of different eGFR formulas[J]. Biomedicines, 2021, 9(10): 1307. DOI: 10.3390/biomedicines9101307. [16] LISBOA PJG, JAYABALAN M, ORTEGA-MARTORELL S, et al. Enhanced survival prediction using explainable artificial intelligence in heart transplantation[J]. Sci Rep, 2022, 12(1): 19525. DOI: 10.1038/s41598-022-23817-2. [17] MEHRA MR, CANTER CE, HANNAN MM, et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: a 10-year update[J]. J Heart Lung Transplant, 2016, 35(1): 1-23. DOI: 10.1016/j.healun.2015.10.023. [18] SINGH TP, GIVERTZ MM, GAUVREAU K. Risk stratification for in-hospital mortality after heart transplantation using the modification of diet in renal disease and the chronic kidney disease epidemiology collaboration equations for estimated glomerular filtration rate[J]. Transplantation, 2014, 98(9): 1000-1006. DOI: 10.1097/TP.0000000000000151. [19] DECATO TW, HAVERKAMP H, HEGEWALD MJ. Cardiopulmonary exercise testing (CPET)[J]. Am J Respir Crit Care Med, 2020, 201(1): P1-P2. DOI: 10.1164/rccm.2011P1. [20] RIETH AJ, RICHTER MJ, TELLO K, et al. Exercise hemodynamics in heart failure patients with preserved and mid-range ejection fraction: key role of the right heart[J]. Clin Res Cardiol, 2022, 111(4): 393-405. DOI: 10.1007/s00392-021-01884-1. [21] CORRÀ U, AGOSTONI PG, ANKER SD, et al. Role of cardiopulmonary exercise testing in clinical stratification in heart failure. a position paper from the Committee on Exercise Physiology and Training of the Heart Failure Association of the European Society of Cardiology[J]. Eur J Heart Fail, 2018, 20(1): 3-15. DOI: 10.1002/ejhf.979. [22] BROCKLEBANK PW, KWON JH, HASHMI ZA, et al. The impact of changes in renal function during waitlist time on outcomes after heart transplantation[J]. J Card Surg, 2022, 37(3): 590-599. DOI: 10.1111/jocs.16188. [23] VIEIRA JL, MEHRA MR. Heart transplantation candidacy[J]. Curr Opin Organ Transplant, 2021, 26(1): 69-76. DOI: 10.1097/MOT.0000000000000828. [24] CLERKIN KJ, SALAKO O, FRIED JA, et al. Impact of temporary percutaneous mechanical circulatory support before transplantation in the 2018 heart allocation system[J]. JACC Heart Fail, 2022, 10(1): 12-23. DOI: 10.1016/j.jchf.2021.08.003. [25] KOBASHIGAWA J, DADHANIA DM, FARR M, et al. Consensus conference on heart-kidney transplantation[J]. Am J Transplant, 2021, 21(7): 2459-2467. DOI: 10.1111/ajt.16512. [26] DANI A, PRICE N, THANGAPPAN K, et al. Heart-kidney listing is better than isolated heart listing for pediatric heart transplant candidates with significant renal insufficiency[J]. J Thorac Cardiovasc Surg, 2022, 164(6): 2019-2031. DOI: 10.1016/j.jtcvs.2021.10.082. [27] ROSSI AP, VELLA JP. Acute kidney disease after liver and heart transplantation[J]. Transplantation, 2016, 100(3): 506-514. DOI: 10.1097/TP.0000000000000916. [28] GARCÍA-GIGORRO R, RENES-CARREÑO E, CORRES PEIRETTI MA, et al. Incidence, risk factors and outcomes of early acute kidney injury after heart transplantation: an 18-year experience[J]. Transplantation, 2018, 102(11): 1901-1908. DOI: 10.1097/TP.0000000000002293. [29] SHOJI S, KUNO T, KOHSAKA S, et al. Incidence and long-term outcome of heart transplantation patients who develop postoperative renal failure requiring dialysis[J]. J Heart Lung Transplant, 2022, 41(3): 356-364. DOI: 10.1016/j.healun.2021.11.017. [30] ROEST S, HESSELINK DA, KLIMCZAK-TOMANIAK D, et al. Incidence of end-stage renal disease after heart transplantation and effect of its treatment on survival[J]. ESC Heart Fail, 2020, 7(2): 533-541. DOI: 10.1002/ehf2.12585. [31] NICOARA A, KRETZER A, COOTER M, et al. Association between primary graft dysfunction and acute kidney injury after orthotopic heart transplantation - a retrospective, observational cohort study[J]. Transpl Int, 2020, 33(8): 887-894. DOI: 10.1111/tri.13615. [32] FORTRIE G, MANINTVELD OC, CALISKAN K, et al. Acute kidney injury as a complication of cardiac transplantation: incidence, risk factors, and impact on 1-year mortality and renal function[J]. Transplantation, 2016, 100(8): 1740-1749. DOI: 10.1097/TP.0000000000000956. [33] MCALISTER FA, EZEKOWITZ J, TARANTINI L, et al. Renal dysfunction in patients with heart failure with preserved versus reduced ejection fraction: impact of the new Chronic Kidney Disease-Epidemiology Collaboration Group formula[J]. Circ Heart Fail, 2012, 5(3): 309-314. DOI: 10.1161/CIRCHEARTFAILURE.111.966242. [34] OTAKI Y, WATANABE T, SHIMIZU M, et al. Association of malnutrition with renal dysfunction and clinical outcome in patients with heart failure[J]. Sci Rep, 2022, 12(1): 16673. DOI: 10.1038/s41598-022-20985-z. [35] ZHOU X, NAKAMURA K, SAHARA N, et al. Exploring and identifying prognostic phenotypes of patients with heart failure guided by explainable machine learning[J]. Life (Basel), 2022, 12(6): 776. DOI: 10.3390/life12060776. -





 下载:
下载: