Effect and mechanism of terminal fucosylation inhibitor on ciclosporin-induced renal epithelial-mesenchymal transition
-
摘要:
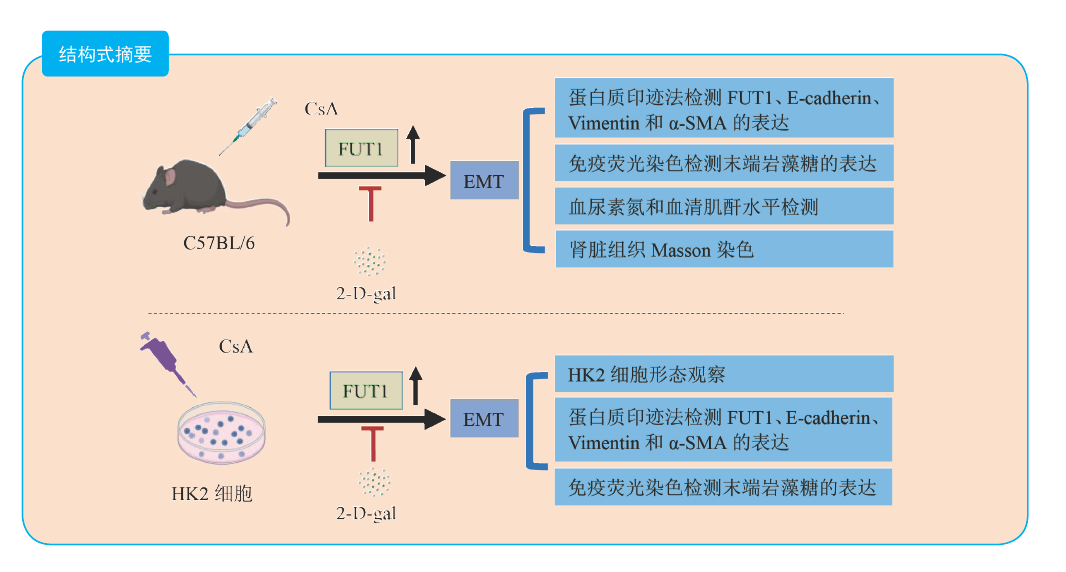
目的 探讨末端岩藻糖基化抑制剂2-脱氧-D-半乳糖(2-D-gal)对环孢素(CsA)诱导的肾脏上皮-间充质转化(EMT)的影响及其机制。 方法 将15只8~10周的雄性C57BL/6小鼠随机分为对照组(Ctrl组)、CsA组和CsA+2-D-gal组,每组各5只。通过蛋白质印迹法检测Ctrl组和CsA组小鼠肾脏组织岩藻糖基转移酶1(FUT1)和EMT相关蛋白E-cadherin、Vimentin、α-平滑肌肌动蛋白(α-SMA)的表达,免疫荧光法检测Ctrl组和CsA组小鼠肾脏组织末端岩藻糖的表达,Masson染色检测各组小鼠肾脏组织纤维化情况,并检测各组小鼠血尿素氮和血清肌酐水平。体外建立CsA诱导肾小管上皮HK2细胞EMT模型,分别用0、2.5、5.0和10.0 μmol/L的CsA刺激HK2细胞24 h,另将HK2细胞分为Ctrl组、2-D-gal组、CsA组和CsA+2-D-gal组,观察不同浓度CsA刺激后及各组HK2细胞形态,通过蛋白质印迹法检测不同浓度CsA刺激后及各组HK2细胞FUT1、E-cadherin、Vimentin和α-SMA的表达,免疫荧光法检测Ctrl组和CsA组HK2细胞末端岩藻糖的表达。 结果 与Ctrl组比较,CsA组小鼠肾脏组织E-cadherin的蛋白相对表达量下降,FUT1、Vimentin和α-SMA蛋白相对表达量均升高(均为P < 0.05),小鼠肾脏组织末端岩藻糖表达增多。与Ctrl组比较,CsA组和CsA+2-D-gal组小鼠的血尿素氮和血清肌酐水平均升高,与CsA组比较,CsA+2-D-gal组小鼠的血尿素氮和血清肌酐水平均降低(均为P < 0.05)。与Ctrl组比较,CsA组和CsA+2-D-gal组小鼠肾脏组织胶原纤维沉积均增多,α-SMA蛋白相对表达量均升高;与CsA组比较,CsA+2-D-gal组小鼠肾脏组织胶原纤维沉积减少,α-SMA蛋白相对表达量下降(均为P < 0.05)。随着CsA的浓度增加,HK2细胞的形态由正常的鹅卵石样逐渐变长变细,HK2细胞FUT1、Vimentin和α-SMA蛋白相对表达量升高,E-cadherin蛋白相对表达量下降,呈浓度依赖性。与Ctrl组比较,CsA组HK2细胞末端岩藻糖表达增多。CsA处理的基础上联合2-D-gal干预后,CsA+2-D-gal组的HK2细胞形态恢复到与正常HK2细胞形态相似。与CsA组比较,CsA+2-D-gal组的HK2细胞E-cadherin蛋白相对表达量升高,Vimentin和α-SMA蛋白相对表达量均下降(均为P < 0.05)。 结论 CsA在体内和体外均可诱导EMT发生,并且伴有末端岩藻糖基化水平增加。2-D-gal可以通过抑制末端岩藻糖基化来抑制CsA诱导的EMT。 -
关键词:
- 2-脱氧-D-半乳糖 /
- 环孢素 /
- 肾毒性 /
- 上皮-间充质转化(EMT) /
- 肾脏纤维化 /
- 岩藻糖基转移酶1(FUT1) /
- 末端岩藻糖 /
- α-平滑肌肌动蛋白(α-SMA)
Abstract:Objective To evaluate the effect and mechanism of terminal fucosylation inhibitor 2-deoxy-D-galactose (2-D-gal) on ciclosporin (CsA)-induced renal epithelial-mesenchymal transition (EMT). Methods Fifteen male C57BL/6 mice aged 8-10 weeks were randomly and evenly divided into the control group (Ctrl group), CsA group and CsA+2-D-gal group (n=5). The expression levels of fucosyltransferase 1 (FUT1), EMT-associated proteins including E-cadherin, Vimentin, α-smooth muscle actin (α-SMA) in the kidney tissues of the Ctrl and CsA groups were detected by Western blot. The expression levels of terminal fucose in the kidney tissues of Ctrl and CsA groups were determined by immunofluorescence. The renal fibrosis of mice in each group was evaluated by Masson staining. The blood urea nitrogen and serum creatinine levels of mice in each group were detected. The in vitro EMT model of renal tubular epithelial cell HK2 was induced by CsA. HK2 cells were stimulated with 0, 2.5, 5.0 and 10.0 μmol/L CsA for 24 h, respectively. In addition, HK2 cells were divided into the Ctrl, 2-D-gal, CsA and CsA+2-D-gal groups. The morphology of HK2 cells after stimulation with different concentrations of CsA and in each group was observed. The expression levels of FUT1, E-cadherin, Vimentin and α-SMA in HK2 cells after stimulation with different concentrations of CsA and in each group were detected by Western blot. The expression level of terminal fucose in HK2 cells of the Ctrl and CsA groups was measured by immunofluorescence. Results Compared with the Ctrl group, the relative expression of E-cadherin protein was down-regulated, those of FUT1, Vimentin and α-SMA proteins were up-regulated (all P < 0.05), and that of terminal fucose in the mouse kidney tissues was up-regulated in the CsA group. Compared with the Ctrl group, the blood urea nitrogen and serum creatinine levels in the CsA and CsA+2-D-gal groups were up-regulated (all P < 0.05). Compared with the CsA group, the blood urea nitrogen and serum creatinine levels in the CsA+2-D-gal group were down-regulated (both P < 0.05). Compared with the Ctrl group, the collagen fiber deposition was increased and the relative expression of α-SMA protein was up-regulated in the mouse kidney tissues of CsA and CsA+2-D-gal groups (all P < 0.05). Compared with the CsA group, the collagen fiber deposition was decreased and the relative expression of α-SMA protein in the mouse kidney tissues was down-regulated in the CsA+2-D-gal group (both P < 0.05). With the increase of CsA concentration, the morphology of HK2 cells gradually became longer and thinner from original normal cobblestone shape, the relative expression levels of FUT1, Vimentin and α-SMA protein in HK2 cells were up-regulated, and that of E-cadherin protein was down-regulated in a concentration-dependent manner. Compared with the Ctrl group, the expression level of terminal fucose of HK2 cells was up-regulated in the CsA group. After CsA treatment combined with 2-D-gal intervention, the morphology of HK2 cells in the CsA+2-D-gal group was restored to resemble that of normal HK2 cells. Compared with the CsA group, the relative expression of E-cadherin protein in HK2 cells was up-regulated, whereas those of Vimentin and α-SMA proteins were down-regulated in the CsA+2-D-gal group (all P < 0.05). Conclusions CsA may induce EMT both in vivo and in vitro, and the terminal fucosylation is increased. 2-D-gal may inhibit CsA-induced EMT by suppressing the terminal fucosylation. -
图 4 CsA对HK2细胞形态、EMT相关蛋白表达和末端岩藻糖基化的影响
注:A图为显微镜下不同浓度CsA作用下的细胞形态图(×200);B图为不同浓度CsA作用下HK2细胞的FUT1、E-cadherin、Vimentin和α-SMA蛋白相对表达量,与0 μmol/L CsA比较,aP < 0.05,与2.5 μmol/L CsA比较,bP < 0.05,与5.0 μmol/L CsA比较,cP < 0.05;C图为Ctrl组和CsA组HK2细胞末端岩藻糖的表达情况(免疫荧光,×200)。
Figure 4. Effects of CsA on morphology of HK2 cells, expression of EMT-related protein and levels of terminal fucosylation
-
[1] PATOCKA J, NEPOVIMOVA E, KUCA K, et al. Cyclosporine A: chemistry and toxicity - a review[J]. Curr Med Chem, 2021, 28(20): 3925-3934. DOI: 10.2174/0929867327666201006153202. [2] STÂRCEA M, GAVRILOVICI C, MUNTEANU M, et al. Focal segmental glomerulosclerosis in children complicated by posterior reversible encephalopathy syndrome[J]. J Int Med Res, 2018, 46(3): 1172-1177. DOI: 10.1177/0300060517746559. [3] LIN CX, LI Y, LIANG S, et al. Metformin attenuates cyclosporine A-induced renal fibrosis in rats[J]. Transplantation, 2019, 103(10): e285-e296. DOI: 10.1097/TP.0000000000002864. [4] CHEN Y, WANG N, YUAN Q, et al. The protective effect of fluorofenidone against cyclosporine A-induced nephrotoxicity[J]. Kidney Blood Press Res, 2019, 44(4): 656-668. DOI: 10.1159/000500924. [5] WELLENS S, DEHOUCK L, CHANDRASEKARAN V, et al. Evaluation of a human iPSC-derived BBB model for repeated dose toxicity testing with cyclosporine A as model compound[J]. Toxicol In Vitro, 2021, 73: 105112. DOI: 10.1016/j.tiv.2021.105112. [6] TAN YC, ABDUL SATTAR M, AHMEDA AF, et al. Apocynin and catalase prevent hypertension and kidney injury in cyclosporine A-induced nephrotoxicity in rats[J]. PLoS One, 2020, 15(4): e0231472. DOI: 10.1371/journal.pone.0231472. [7] PHAN K, CHARLTON O, BAKER C, et al. Dermatologist attitudes toward ciclosporin use in atopic dermatitis[J]. J Dermatolog Treat, 2021, 32(8): 922-924. DOI: 10.1080/09546634.2020.1724251. [8] HOŠKOVÁ L, MÁLEK I, KOPKAN L, et al. Pathophysiological mechanisms of calcineurin inhibitor-induced nephrotoxicity and arterial hypertension[J]. Physiol Res, 2017, 66(2): 167-180. DOI: 10.33549/physiolres.933332. [9] PONTICELLI C, GLASSOCK RJ. Prevention of complications from use of conventional immunosuppressants: a critical review[J]. J Nephrol, 2019, 32(6): 851-870. DOI: 10.1007/s40620-019-00602-5. [10] NAGAVALLY RR, SUNILKUMAR S, AKHTAR M, et al. Chrysin ameliorates cyclosporine-A-induced renal fibrosis by inhibiting TGF-β1-induced epithelial-mesenchymal transition[J]. Int J Mol Sci, 2021, 22(19): 10252. DOI: 10.3390/ijms221910252. [11] WU Q, WANG X, NEPOVIMOVA E, et al. Mechanism of cyclosporine A nephrotoxicity: oxidative stress, autophagy, and signalings[J]. Food Chem Toxicol, 2018, 118: 889-907. DOI: 10.1016/j.fct.2018.06.054. [12] GROENENDYK J, ROBINSON A, WANG Q, et al. Tauroursodeoxycholic acid attenuates cyclosporine-induced renal fibrogenesis in the mouse model[J]. Biochim Biophys Acta Gen Subj, 2019, 1863(7): 1210-1216. DOI: 10.1016/j.bbagen.2019.04.016. [13] FANG M, KANG L, WANG X, et al. Inhibition of core fucosylation limits progression of diabetic kidney disease[J]. Biochem Biophys Res Commun, 2019, 520(3): 612-618. DOI: 10.1016/j.bbrc.2019.10.037. [14] YU A, ZHAO J, ZHONG J, et al. Altered O-glycomes of renal brush-border membrane in model rats with chronic kidney diseases[J]. Biomolecules, 2021, 11(11): 1560. DOI: 10.3390/biom11111560. [15] LIU A, WANG X, HU X, et al. Core fucosylation involvement in the paracrine regulation of proteinuria-induced renal interstitial fibrosis evaluated with the use of a microfluidic chip[J]. Acta Biomater, 2022, 142: 99-112. DOI: 10.1016/j.actbio.2022.02.020. [16] LI J, HSU HC, MOUNTZ JD, et al. Unmasking fucosylation: from cell adhesion to immune system regulation and diseases[J]. Cell Chem Biol, 2018, 25(5): 499-512. DOI: 10.1016/j.chembiol.2018.02.005. [17] MEHTA K, PATEL K, PANDYA S, et al. Altered mRNA expression of fucosyltransferases and fucosidase predicts prognosis in human oral carcinoma[J]. Int J Mol Cell Med, 2021, 10(2): 123-131. DOI: 10.22088/IJMCM.BUMS.10.2.123. [18] PARK S, LIM JM, CHUN JN, et al. Altered expression of fucosylation pathway genes is associated with poor prognosis and tumor metastasis in non small cell lung cancer[J]. Int J Oncol, 2020, 56(2): 559-567. DOI: 10.3892/ijo.2019.4953. [19] LAI TY, CHEN IJ, LIN RJ, et al. Fucosyltransferase 1 and 2 play pivotal roles in breast cancer cells[J]. Cell Death Discov, 2019, 5: 74. DOI: 10.1038/s41420-019-0145-y. [20] LOONG JH, WONG TL, TONG M, et al. Glucose deprivation-induced aberrant FUT1-mediated fucosylation drives cancer stemness in hepatocellular carcinoma[J]. J Clin Invest, 2021, 131(11): e143377. DOI: 10.1172/JCI143377. [21] HAN C, JIANG YH, LI W, et al. Astragalus membranaceus and salvia miltiorrhiza ameliorates cyclosporin A-induced chronic nephrotoxicity through the "gut-kidney axis"[J]. J Ethnopharmacol, 2021, 269: 113768. DOI: 10.1016/j.jep.2020.113768. [22] XIA Z, ZHANG C, GUO C, et al. Nanoformulation of a carbon monoxide releasing molecule protects against cyclosporin A-induced nephrotoxicity and renal fibrosis via the suppression of the NLRP3 inflammasome mediated TGF-β/Smad pathway[J]. Acta Biomater, 2022, 144: 42-53. DOI: 10.1016/j.actbio.2022.03.024. [23] LI R, GUO Y, ZHANG Y, et al. Salidroside ameliorates renal interstitial fibrosis by inhibiting the TLR4/NF-κB and MAPK signaling pathways[J]. Int J Mol Sci, 2019, 20(5): 1103. DOI: 10.3390/ijms20051103. [24] BAI Y, WANG W, YIN P, et al. Ruxolitinib alleviates renal interstitial fibrosis in UUO mice[J]. Int J Biol Sci, 2020, 16(2): 194-203. DOI: 10.7150/ijbs.39024. [25] GENG XQ, MA A, HE JZ, et al. Ganoderic acid hinders renal fibrosis via suppressing the TGF-β/Smad and MAPK signaling pathways[J]. Acta Pharmacol Sin, 2020, 41(5): 670-677. DOI: 10.1038/s41401-019-0324-7. [26] HUANG J, YAO X, WENG G, et al. Protective effect of curcumin against cyclosporine A induced rat nephrotoxicity[J]. Mol Med Rep, 2018, 17(4): 6038-6044. DOI: 10.3892/mmr.2018.8591. [27] FUJITA K, HATANO K, HASHIMOTO M, et al. Fucosylation in urological cancers[J]. Int J Mol Sci, 2021, 22(24): 13333. DOI: 10.3390/ijms222413333. [28] SHAN M, YANG D, DOU H, et al. Fucosylation in cancer biology and its clinical applications[J]. Prog Mol Biol Transl Sci, 2019, 162: 93-119. DOI: 10.1016/bs.pmbts.2019.01.002. [29] ZHANG B, CHEN X, RU F, et al. Liproxstatin-1 attenuates unilateral ureteral obstruction-induced renal fibrosis by inhibiting renal tubular epithelial cells ferroptosis[J]. Cell Death Dis, 2021, 12(9): 843. DOI: 10.1038/s41419-021-04137-1. [30] SHEN H, HE Q, DONG Y, et al. Upregulation of miRNA-1228-3p alleviates TGF-β-induced fibrosis in renal tubular epithelial cells[J]. Histol Histopathol, 2020, 35(10): 1125-1133. DOI: 10.14670/HH-18-242. [31] DENG G, CHEN L, ZHANG Y, et al. Fucosyltransferase 2 induced epithelial-mesenchymal transition via TGF-β/Smad signaling pathway in lung adenocarcinaoma[J]. Exp Cell Res, 2018, 370(2): 613-622. DOI: 10.1016/j.yexcr.2018.07.026. -





 下载:
下载: