-
摘要: 随着科学技术的迅速发展,通过干细胞与组织工程的新技术来再造人类肝脏,为解决供肝短缺提供了新的思路,是未来科学研究的重点。本文从去细胞化基质支架构建肝脏类器官、生物材料微型模具构建肝脏类器官、细胞诱导的无支架培养构建肝脏类器官、构建嵌合体制备人工肝脏等方面介绍肝脏组织工程研究的最新进展。
-
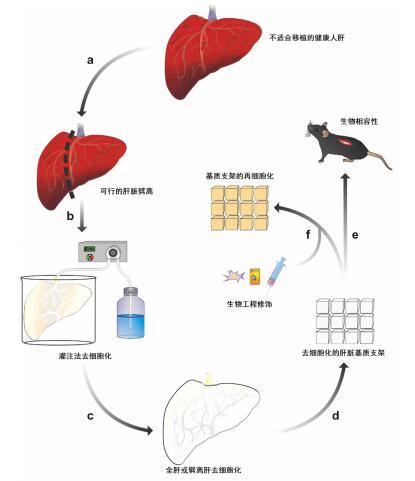
图 1 基于肝脏基质支架的培养方法[4]
Figure 1. Culture method based on liver matrix scaffold
图 2 基于微型模具制作新型肝脏“组织种子”[20]
Figure 2. Fabrication of new liver tissue seed based on micro mold
图 3 人猪嵌合胚胎的培育[43]
Figure 3. Cultivation of human and porcine chimeric embryos
-
[1] Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2015 annual data report: liver[J]. Am J Transplant, 2017, 17 (Suppl 1): 174-251. DOI: 10.1111/ajt.14126. [2] Wang Y, Nicolas CT, Chen HS, et al. Recent advances in decellularization and recellularization for tissue-engineered liver grafts[J]. Cells Tissues Organs, 2017, 203(4): 203-214. DOI: 10.1159/000452761. [3] Caralt M. Present and future of regenerative medicine: liver transplantation[J]. Transplant Proc, 2015, 47(8): 2377-2379. DOI: 10.1016/j.transproceed.2015.08.029. [4] Mazza G, Rombouts K, Rennie Hall A, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation[J]. Sci Rep, 2015, 5: 13079. DOI: 10.1038/srep13079. [5] Baptista PM, Siddiqui MM, Lozier G, et al. The use of whole organ decellularization for the generation of a vascularized liver organoid[J]. Hepatology, 2011, 53(2): 604-617. DOI: 10.1002/hep.24067. [6] Hassanein W, Uluer MC, Langford J, et al. Recellularization via the bile duct supports functional allogenic and xenogenic cell growth on a decellularized rat liver scaffold[J]. Organogenesis, 2017, 13(1): 16-27. DOI: 10.1080/15476278.2016.1276146. [7] Ko IK, Peng L, Peloso A, et al. Bioengineered transplantable porcine livers with re-endothelialized vasculature[J]. Biomaterials, 2015, 40: 72-79. DOI: 10.1016/j.biomaterials.2014.11.027. [8] Soto-Gutierrez A, Zhang L, Medberry C, et al. A whole-organ regenerative medicine approach for liver replacement[J]. Tissue Eng Part C Methods, 2011, 17(6): 677-686. DOI: 10.1089/ten.tec.2010.0698. [9] Uygun BE, Soto-Gutierrez A, Yagi H, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix[J]. Nat Med, 2010, 16(7): 814-820. DOI: 10.1038/nm.2170. [10] Yagi H, Fukumitsu K, Fukuda K, et al. Human-scale whole-organ bioengineering for liver transplantation: a regenerative medicine approach[J]. Cell Transplant, 2013, 22(2): 231-242. DOI: 10.3727/096368912X654939. [11] Lin P, Chan WC, Badylak SF, et al. Assessing porcine liver-derived biomatrix for hepatic tissue engineering[J]. Tissue Eng, 2004, 10(7/8): 1046-1053. DOI: 10.1089/ten.2004.10.1046 [12] Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes[J]. Biomaterials, 2011, 32(12): 3233-3243. DOI: 10.1016/j.biomaterials.2011.01.057. [13] Conklin BS, Wu H, Lin PH, et al. Basic fibroblast growth factor coating and endothelial cell seeding of a decellularized heparin-coated vascular graft[J]. Artif Organs, 2004, 28(7): 668-675. DOI: 10.1111/j.1525-1594.2004.00062.x. [14] Nakamura S, Ijima H. Solubilized matrix derived from decellularized liver as a growth factor-immobilizable scaffold for hepatocyte culture[J]. J Biosci Bioeng, 2013, 116(6): 746-753. DOI: 10.1016/j.jbiosc.2013.05.031. [15] Uygun BE, Yarmush ML, Uygun K. Application of whole-organ tissue engineering in hepatology[J]. Nat Rev Gastroenterol Hepatol, 2012, 9(12): 738-744. DOI: 10.1038/nrgastro.2012.140. [16] Verhulsel M, Vignes M, Descroix S, et al. A review of microfabrication and hydrogel engineering for micro-organs on chips[J]. Biomaterials, 2014, 35(6): 1816-1832. DOI: 10.1016/j.biomaterials.2013.11.021. [17] Yamada M, Utoh R, Ohashi K, et al. Controlled formation of heterotypic hepatic micro-organoids in anisotropic hydrogel microfibers for long-term preservation of liver-specific functions[J]. Biomaterials, 2012, 33(33):8304-8315. DOI: 10.1016/j.biomaterials.2012.07.068. [18] Rennert K, Steinborn S, Gröger M, et al. A microfluidically perfused three dimensional human liver model[J]. Biomaterials, 2015, 71:119-131. DOI: 10.1016/j.biomaterials.2015.08.043. [19] Chen AA, Thomas DK, Ong LL, et al. Humanized mice with ectopic artificial liver tissues[J]. Proc Natl Acad Sci USA, 2011, 108(29): 11842-11847. DOI: 10.1073/pnas.1101791108. [20] Stevens KR, Scull MA, Ramanan V, et al. In situ expansion of engineered human liver tissue in a mouse model of chronic liver disease[J]. Sci Transl Med, 2017, 9(399): eaah5505. DOI: 10.1126/scitranslmed.aah5505. [21] Coghlan A. 3D printer makes tiniest human liver ever[EB/OL]. (2013-04-23). https://www.newscientist.com/article/dn23419-3d-printer-makes-tiniest-human-liver-ever.html. [22] Sasai Y. Next-generation regenerative medicine: organogenesis from stem cells in 3D culture[J]. Cell Stem Cell, 2013, 12(5): 520-530. DOI: 10.1016/j.stem.2013.04.009. [23] Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors[J]. Cell, 2007, 131(5): 861-872. DOI: 10.1016/j.cell.2007.11.019. [24] Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function[J]. Science, 2001, 294(5542): 559-563. DOI: 10.1126/science.1063889. [25] Korzh S, Pan X, Garcia-Lecea M, et al. Requirement of vasculogenesis and blood circulation in late stages of liver growth in zebrafish[J]. BMC Dev Biol, 2008, 8:84. DOI: 10.1186/1471-213X-8-84. [26] Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant[J]. Nature, 2013, 499(7459): 481-484. DOI: 10.1038/nature12271. [27] Takebe T, Enomura M, Yoshizawa E, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation[J]. Cell Stem Cell, 2015, 16(5): 556-565. DOI: 10.1016/j.stem.2015.03.004. [28] Guye P, Ebrahimkhani MR, Kipniss N, et al. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using GATA6[J]. Nat Commun, 2016, 7:10243. DOI: 10.1038/ncomms10243. [29] Ozair MZ, Noggle S, Warmflash A, et al. SMAD7 directly converts human embryonic stem cells to telencephalic fate by a default mechanism[J]. Stem Cells, 2013, 31(1): 35-47. DOI: 10.1002/stem.1246. [30] Peterkin T, Gibson A, Patient R. GATA-6 maintains BMP-4 and Nkx2 expression during cardiomyocyte precursor maturation[J]. EMBO J, 2003, 22(16): 4260-4273. DOI: 10.1093/emboj/cdg400. [31] Rhim JA, Sandgren EP, Palmiter RD, et al. Complete reconstitution of mouse liver with xenogeneic hepatocytes[J]. Proc Natl Acad Sci USA, 1995, 92(11): 4942-4946. doi: 10.1073/pnas.92.11.4942 [32] Dandri M, Burda MR, Török E, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis B virus[J]. Hepatology, 2001, 33(4): 981-988. DOI: 10.1053/jhep.2001.23314. [33] Tateno C, Yoshizane Y, Saito N, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs[J]. Am J Pathol, 2004, 165(3): 901-912. DOI: 10.1016/S0002-9440(10)63352-4. [34] Meuleman P, Libbrecht L, De Vos R, et al. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera[J]. Hepatology, 2005, 41(4): 847-856. DOI: 10.1002/hep.20657. [35] Azuma H, Paulk N, Ranade A, et al. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/-mice[J]. Nat Biotechnol, 2007, 25(8): 903-910. DOI: 10.1038/nbt1326. [36] Bissig KD, Le TT, Woods NB, et al. Repopulation of adult and neonatal mice with human hepatocytes: a chimeric animal model[J]. Proc Natl Acad Sci USA, 2007, 104(51): 20507-20511. DOI: 10.1073/pnas.0710528105. [37] Rashid T, Kobayashi T, Nakauchi H. Revisiting the flight of icarus: making human organs from PSCs with large animal chimeras[J]. Cell Stem Cell, 2014, 15(4): 406-409. DOI: 10.1016/j.stem.2014.09.013. [38] Kim H, Kim JS. A guide to genome engineering with programmable nucleases[J]. Nat Rev Genet, 2014, 15(5): 321-334. DOI: 10.1038/nrg3686. [39] Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering[J]. Cell, 2014, 157(6): 1262-1278. DOI: 10.1016/j.cell.2014.05.010. [40] Yang L, Güell M, Niu D, et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs)[J]. Science, 2015, 350(6264): 1101-1104. DOI: 10.1126/science.aad1191. [41] Cooper DK, Ekser B, Ramsoondar J, et al. The role of genetically engineered pigs in xenotransplantation research[J]. J Pathol, 2016, 238(2): 288-299. DOI: 10.1002/path.4635. [42] Shaw D, Dondorp W, Geijsen N, et al. Creating human organs in chimaera pigs: an ethical source of immunocompatible organs?[J]. J Med Ethics, 2015, 41(12): 970-974. DOI: 10.1136/medethics-2014-102224. [43] Wu J, Platero-Luengo A, Sakurai M, et al. Interspecies chimerism with mammalian pluripotent stem cells[J]. Cell, 2017, 168(3): 473-486. DOI: 10.1016/j.cell.2016.12.036. [44] Xiang AP, Mao FF, Li WQ, et al. Extensive contribution of embryonic stem cells to the development of an evolutionarily divergent host[J]. Hum Mol Genet, 2008, 17(1): 27-37. DOI: 10.1093/hmg/ddm282. [45] Li W, Huang L, Lin W, et al. Engraftable neural crest stem cells derived from cynomolgus monkey embryonic stem cells[J]. Biomaterials, 2015, 39: 75-84. DOI: 10.1016/j.biomaterials.2014.10.056. -





 下载:
下载: