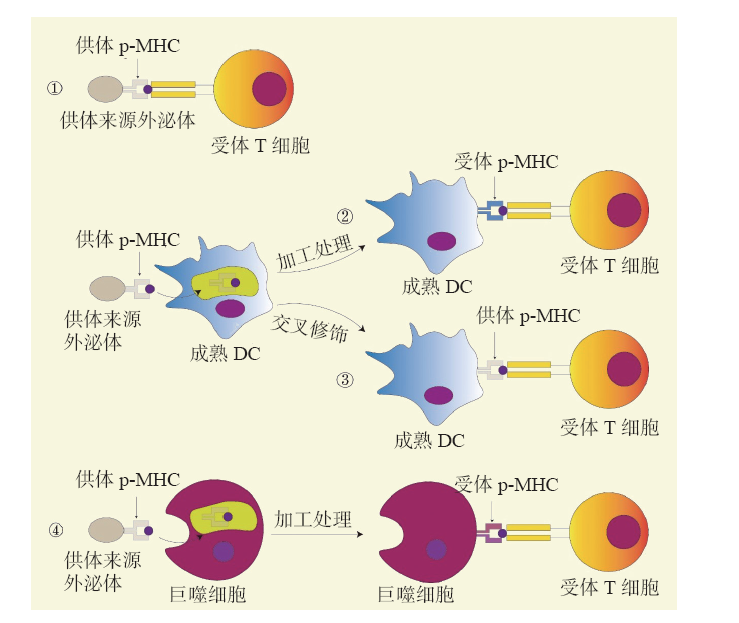
-
摘要: 肺移植术后排斥反应包括急性排斥反应(AR)和以慢性移植肺功能障碍(CLAD)为主要表现的慢性排斥反应,是影响同种异体移植物长期存活的主要因素。外泌体是真核生物细胞间通讯的一种细胞外纳米囊泡,可以携带复杂生物学信息,参与各种生理、病理过程,已成为排斥反应中的重要免疫介质,通过多种途径调控排斥反应的发生发展,在排斥反应的监测和治疗中亦发挥着关键作用。本文就肺移植术后排斥反应的类型、外泌体调控排斥反应的机制、外泌体作为生物标志物及其在排斥反应治疗中的应用做一综述,旨在为肺移植术后排斥反应的综合诊治提供新的方向。
-
关键词:
- 肺移植 /
- 外泌体 /
- 慢性移植肺功能障碍(CLAD) /
- 急性细胞介导排斥反应(ACR) /
- 限制性移植物综合征(RAS) /
- 闭塞性细支气管炎综合征(BOS) /
- 主要组织相容性复合体(MHC) /
- 供者特异性抗体(DSA)
Abstract: Rejection after lung transplantation, including acute rejection (AR) and chronic rejection manifested with chronic lung allograft dysfunction (CLAD), is the main factor affecting the long-term survival of allografts. Exosome, a type of extracellular nanovesicle for intercellular communication among eukaryotic cells, could carry complex biological information and participate in various physiological and pathological processes. Exosome has become a critical immune medium in rejection, regulates the incidence and development of rejection through multiple pathways, and also plays a key role in the monitoring and management of rejection. In this article, the type of rejection after lung transplantation, the mechanism underlying the role of exosome in regulating rejection, exosome acting as biomarkers and the application in rejection treatment were reviewed, aiming to provide a novel direction for comprehensive diagnosis and treatment of rejection following lung transplantation. -
表 1 外泌体标志物在肺移植术后不同状态下的表达情况
Table 1. Expression of exosome markers in different conditions after lung transplantation
外泌体标志物 类别 稳定状态 AR RAS BOS 临床意义 CⅡTA[4] MHCⅡ型 不表达 表达 高表达 中表达 介导适应性免疫应答及T细胞的选择活化过程 20S蛋白酶体[12] 蛋白酶体 低表达 表达 高表达 中表达 参与适应性免疫反应,介导抗原提呈过程,促进NF-κB生成 CD80[18] B7 不表达 表达 表达 表达 协同刺激T细胞增殖,诱导免疫应答,介导细胞毒反应 CD86[18] 不表达 表达 表达 表达 CD40[18] TNF或TNFR① 不表达 表达 表达 表达 促进细胞因子和趋化因子产生,诱导共刺激分子表达,促进抗原的交叉提呈 Ⅴ型胶原[20] 自身抗原 不表达 表达 高表达 高表达 诱导细胞免疫反应,促进自身抗体形成 K-α1微管蛋白[20] 不表达 表达 中表达 高表达 HIF-1α[29] HIF-1 不表达 表达 表达 表达 参与能量代谢、血管生成,增强对缺氧环境的代谢适应性 IRAK1[29] TLR 不表达 表达 表达 表达 参与免疫识别,调控细胞因子分泌,影响适应性免疫应答 MyD88[29] 不表达 表达 表达 表达 NF-κB[29] 转录因子 不表达 表达 高表达 中表达 介导炎症反应,参与免疫应答 miR-144[30] miRNA 不表达 不表达 不表达 高表达 诱导炎症反应、内皮激活、AMR和Th17分化 PIGR[32] 糖蛋白 低表达 表达 高表达 中表达 结合并运输聚合免疫球蛋白至黏膜表面,引起内皮活化,诱导免疫应答 HLA-DQ[32] HLAⅡ型 低表达 表达 高表达 中表达 将抗原提呈给CD 4+T细胞,促进T细胞增殖,刺激B细胞产生体液免疫 HLA-DR[32] 低表达 表达 高表达 中表达 注:①TNF或TNFR为肿瘤坏死因子或肿瘤坏死因子受体。 -
[1] AIGNER C. Current developments in lung transplantation[J]. Pathologe, 2019, 40(Suppl 3): 363-365. DOI: 10.1007/s00292-019-00690-x. [2] CHUNG PA, DILLING DF. Immunosuppressive strategies in lung transplantation[J]. Ann Transl Med, 2020, 8(6): 409. DOI: 10.21037/atm.2019.12.117. [3] VAN DER MARK SC, HOEK RAS, HELLEMONS ME. Developments in lung transplantation over the past decade[J]. Eur Respir Rev, 2020, 29(157): 190132. DOI: 10.1183/16000617.0132-2019. [4] MOHANAKUMAR T, SHARMA M, BANSAL S, et al. A novel mechanism for immune regulation after human lung transplantation[J]. J Thorac Cardiovasc Surg, 2019, 157(5): 2096-2106. DOI: 10.1016/j.jtcvs.2018.12.105. [5] 姚培学, 郭小旭, 贺艳飞, 等. 外泌体microRNAs作为肺部疾病诊断性生物标志物的研究进展[J]. 实用医学杂志, 2020, 36(13): 1839-1843. DOI: 10.3969/j.issn.1006-5725.2020.13.030.YAO PX, GUO XX, HE YF, et al. The research progress of the exosomal microRNAs as biomarkers of lung diseases[J]. J Pract Med, 2020, 36(13): 1839-1843. DOI: 10.3969/j.issn.1006-5725.2020.13.030. [6] HSIAO HM, SCOZZI D, GAUTHIER JM, et al. Mechanisms of graft rejection after lung transplantation[J]. Curr Opin Organ Transplant, 2017, 22(1): 29-35. DOI: 10.1097/MOT.0000000000000371. [7] PARULEKAR AD, KAO CC. Detection, classification, and management of rejection after lung transplantation[J]. J Thorac Dis, 2019, 11(Suppl 14): S1732-S1739. DOI: 10.21037/jtd.2019.03.83. [8] 中华医学会器官移植学分会. 中国肺移植免疫抑制治疗及排斥反应诊疗规范(2019版)[J/CD]. 中华移植杂志(电子版), 2019, 13(2): 94-98. DOI: 10.3877/cma.j.issn.1674-3903.2019.02.004.Branch of Organ Transplantation of Chinese Medical Association. Diagnosis and treatment specification for immunosuppressive therapy and rejection of lung transplantation in China (2019 edition)[J/CD]. Chin J Transplant (Electr Edit), 2019, 13(2): 94-98. DOI: 10.3877/cma.j.issn.1674-3903.2019.02.004. [9] 张稷. 2016国际心肺移植协会共识: 肺移植术后抗体介导的排斥反应[J/CD]. 实用器官移植电子杂志, 2017, 5(5): 321-328. DOI: 10.3969/j.issn.2095-5332.2017.05.001.ZHANG J. 2016 International Heart and Lung Transplantation Association consensus: antibody-mediated rejection after lung transplantation[J/CD]. Pract J Organ Transplant (Electr Vers), 2017, 5(5): 321-328. DOI: 10.3969/j.issn.2095-5332.2017.05.001. [10] NEUHAUS K, HOHLFELDER B, BOLLINGER J, et al. Antibody-mediated rejection management following lung transplantation[J]. Ann Pharmacother, 2022, 56(1): 60-64. DOI: 10.1177/10600280211012410. [11] VERLEDEN GM, VOS R, VANAUDENAERDE B, et al. Current views on chronic rejection after lung transplantation[J]. Transpl Int, 2015, 28(10): 1131-1139. DOI: 10.1111/tri.12579. [12] SURESHBABU A, FLEMING T, MOHANAKUMAR T. Autoantibodies in lung transplantation[J]. Transpl Int, 2020, 33(1): 41-49. DOI: 10.1111/tri.13487. [13] BENDEN C, HAUGHTON M, LEONARD S, et al. Therapy options for chronic lung allograft dysfunction-bronchiolitis obliterans syndrome following first-line immunosuppressive strategies: a systematic review[J]. J Heart Lung Transplant, 2017, 36(9): 921-933. DOI: 10.1016/j.healun.2017.05.030. [14] MIRZAKHANI M, MOHAMMADNIA-AFROUZI M, SHAHBAZI M, et al. The exosome as a novel predictive/diagnostic biomarker of rejection in the field of transplantation[J]. Clin Immunol, 2019, 203: 134-141. DOI: 10.1016/j.clim.2019.04.010. [15] KONSTANTINOV IE, YONG MS. Exosome signaling: a ubiquitous process in rejection and regeneration?[J]. J Thorac Cardiovasc Surg, 2018, 155(6): 2490-2491. DOI: 10.1016/j.jtcvs.2018.01.021. [16] PEGTEL DM, GOULD SJ. Exosomes[J]. Annu Rev Biochem, 2019, 88: 487-514. DOI: 10.1146/annurev-biochem-013118-111902. [17] GREGSON AL, HOJI A, INJEAN P, et al. Altered exosomal RNA profiles in bronchoalveolar lavage from lung transplants with acute rejection[J]. Am J Respir Crit Care Med, 2015, 192(12): 1490-1503. DOI: 10.1164/rccm.201503-0558OC. [18] RAVICHANDRAN R, BANSAL S, RAHMAN M, et al. The role of donor-derived exosomes in lung allograft rejection[J]. Hum Immunol, 2019, 80(8): 588-594. DOI: 10.1016/j.humimm.2019.03.012. [19] SHARMA M, RAVICHANDRAN R, BANSAL S, et al. Tissue-associated self-antigens containing exosomes: role in allograft rejection[J]. Hum Immunol, 2018, 79(9): 653-658. DOI: 10.1016/j.humimm.2018.06.005. [20] GUNASEKARAN M, XU Z, NAYAK DK, et al. Donor-derived exosomes with lung self-antigens in human lung allograft rejection[J]. Am J Transplant, 2017, 17(2): 474-484. DOI: 10.1111/ajt.13915. [21] GONZALEZ-NOLASCO B, WANG M, PRUNEVIEILLE A, et al. Emerging role of exosomes in allorecognition and allograft rejection[J]. Curr Opin Organ Transplant, 2018, 23(1): 22-27. DOI: 10.1097/MOT.0000000000000489. [22] HWANG B, BRYERS J, MULLIGAN MS. Potential role of exosome-based allorecognition pathways involved in lung transplant rejection[J]. J Thorac Cardiovasc Surg, 2021, 161(2): e129-e134. DOI: 10.1016/j.jtcvs.2020.04.183. [23] MATTKE J, VASU S, DARDEN CM, et al. Role of exosomes in islet transplantation[J]. Front Endocrinol (Lausanne), 2021, 12: 681600. DOI: 10.3389/fendo.2021.681600. [24] LIU Q, ROJAS-CANALES DM, DIVITO SJ, et al. Donor dendritic cell-derived exosomes promote allograft-targeting immune response[J]. J Clin Invest, 2016, 126(8): 2805-2820. DOI: 10.1172/JCI84577. [25] BURLINGHAM WJ. Exosomes: the missing link between microchimerism and acquired tolerance?[J]. Chimerism, 2014, 5(3/4): 63-67. DOI: 10.1080/19381956.2015.1082026. [26] ELASHIRY M, ELSAYED R, CUTLER CW. Exogenous and endogenous dendritic cell-derived exosomes: lessons learned for immunotherapy and disease pathogenesis[J]. Cells, 2021, 11(1): 115. DOI: 10.3390/cells11010115. [27] PANG XL, WANG ZG, LIU L, et al. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation[J]. Aging (Albany NY), 2019, 11(20): 8911-8924. DOI: 10.18632/aging.102346. [28] LEMA DA, BURLINGHAM WJ. Role of exosomes in tumour and transplant immune regulation[J]. Scand J Immunol, 2019, 90(5): e12807. DOI: 10.1111/sji.12807. [29] GUNASEKARAN M, SHARMA M, HACHEM R, et al. Circulating exosomes with distinct properties during chronic lung allograft rejection[J]. J Immunol, 2018, 200(8): 2535-2541. DOI: 10.4049/jimmunol.1701587. [30] RAHMAN M, SURESHBABU A, TOKMAN S, et al. Chronic lung allograft dysfunction: immune responses induced by circulating exosomes with lung-associated self-antigens[J]. Crit Rev Immunol, 2019, 39(2): 123-134. DOI: 10.1615/CritRevImmunol.2019030635. [31] VALLABHAJOSYULA P, KORUTLA L, HABERTHEUER A, et al. Tissue-specific exosome biomarkers for noninvasively monitoring immunologic rejection of transplanted tissue[J]. J Clin Invest, 2017, 127(4): 1375-1391. DOI: 10.1172/JCI87993. [32] BANSAL S, ARJUNA A, PERINCHERI S, et al. Restrictive allograft syndrome vs bronchiolitis obliterans syndrome: immunological and molecular characterization of circulating exosomes[J]. J Heart Lung Transplant, 2022, 41(1): 24-33. DOI: 10.1016/j.healun.2021.09.001. [33] HABERTHEUER A, RAM C, SCHMIERER M, et al. Circulating donor lung-specific exosome profiles enable noninvasive monitoring of acute rejection in a rodent orthotopic lung transplantation model[J]. Transplantation, 2022, 106(4): 754-756. DOI: 10.1097/TP.0000000000003820. [34] MONGUIÓ-TORTAJADA M, LAUZURICA-VALDEMOROS R, BORRÀS FE. Tolerance in organ transplantation: from conventional immunosuppression to extracellular vesicles[J]. Front Immunol, 2014, 5: 416. DOI: 10.3389/fimmu.2014.00416. [35] LEE BC, KANG I, YU KR. Therapeutic features and updated clinical trials of mesenchymal stem cell (MSC)-derived exosomes[J]. J Clin Med, 2021, 10(4): 711. DOI: 10.3390/jcm10040711. [36] RAHMATI S, SHOJAEI F, SHOJAEIAN A, et al. An overview of current knowledge in biological functions and potential theragnostic applications of exosomes[J]. Chem Phys Lipids, 2020, 226: 104836. DOI: 10.1016/j.chemphyslip.2019.104836. [37] YU X, HUANG C, SONG B, et al. CD4+CD25+regulatory T cells-derived exosomes prolonged kidney allograft survival in a rat model[J]. Cell Immunol, 2013, 285(1/2): 62-68. DOI: 10.1016/j.cellimm.2013.06.010. -





 下载:
下载: