Clinical diagnosis and treatment guidelines for cytomegalovirus infection in kidney transplant recipients in China (2023 edition)
-
摘要: 近几年在实体器官移植(SOT)受者巨细胞病毒(CMV)感染诊疗领域,无论是诊断方法还是新型抗CMV药物都有了一些新的进展,对CMV感染的诊治产生了积极的影响。为了进一步规范中国肾移植术后CMV感染的管理,中华医学会器官移植学分会组织了国内多个学科相关领域专家,参考《中国实体器官移植受者巨细胞病毒感染诊疗指南(2016版)》和国内外已发表的最新文献和指南,制定了《中国肾移植受者巨细胞病毒感染诊疗指南(2023版)》,新版指南更新了CMV流行病学,CMV感染的危险因素和普遍性预防的研究进展,新增CMV感染定义,细化CMV血症和CMV病的诊断标准,并对新型抗CMV药物进行了介绍。Abstract: In recent years, there have been significant advances in the diagnosis and treatment of cytomegalovirus (CMV) infection in solid organ transplant (SOT) recipients, including diagnostic method and anti-CMV drugs. These advancements have had a positive impact on the management of CMV infection in SOT recipients. To further standardize the management of CMV infection after kidney transplantation in China, Branch of Organ Transplantation of Chinese Medical Association organized a multidisciplinary group of experts in relevant fields. They referred to the ‘Diagnosis and Treatment Guidelines for Cytomegalovirus Infection in Solid Organ Transplant Recipients in China (2016 edition)’ and the latest published literature and guidelines, resulting in the development of the ‘Clinical Diagnosis and Treatment Guidelines for Cytomegalovirus Infection in Kidney Transplant Recipients in China (2023 edition)’. The updated guideline includes CMV epidemiology, research progress on the risk factors and universal prevention of CMV infection, the definition for CMV infection, detailed diagnostic criteria for CMV viremia and CMV disease, as well as an introduction to new anti-CMV drugs.
-
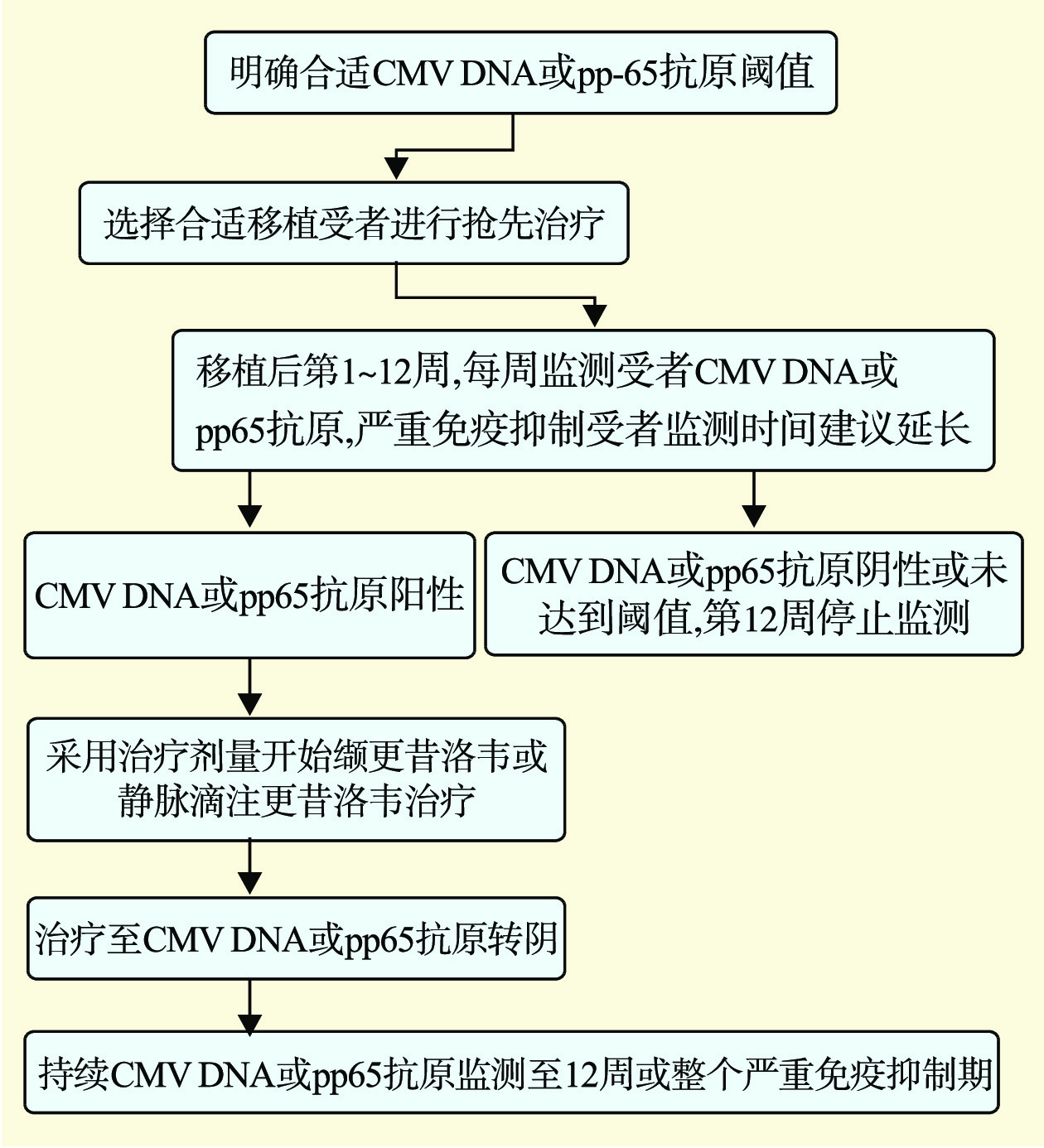
图 2 临床对于SOT受者耐药CMV的诊治流程
注:GCV为更昔洛韦;VGCV为缬更昔洛韦;FOS为膦甲酸钠;CDV为西多福韦;EC50为半最大效应浓度。a为6周内少见;b为症状无改善或病毒载量不降;c为根据肾功能选择足量更昔洛韦(5 mg/kg,静脉注射,每日2次)或高剂量更昔洛韦(10 mg/kg,静脉注射,每日2次);d为包括导致<2倍EC50变化的序列变异;e为根据高剂量更昔洛韦成功治疗病例报告,更昔洛韦浓度为5~10倍EC50;f为西多福韦有效性临床研究数据有限,高剂量更昔洛韦是部分突变类型的治疗选择。
Figure 2. Clinical diagnosis and treatment process for drug-resistant CMV in SOT recipients
表 1 牛津大学证据分级与推荐意见强度分级标准
Table 1. Level of evidence and recommended grades of Oxford University
推荐强度 证据级别 描述 A 1a 随机对照试验的系统评价 1b 结果可信区间小的随机对照试验 1c 显示“全或无效应”的任何证据 B 2a 队列研究的系统评价 2b 单个的队列研究(包括低质量的随机对照试验,如失访率>20%) 2c 基于患者结局的研究 3a 病例对照研究的系统评价 3b 单个病例对照研究 C 4 病例系列报告、低质量队列研究和低质量病例对照研究 D 5 专家意见(即无临床研究支持的仅依据基础研究或临床经验的推测) 表 2 CMV感染和CMV病的定义
Table 2. Definition of CMV infection and CMV disease
类别 确诊 临床诊断 CMV综合征 未定义 经病毒分离、快速培养、抗原血症或NAT检测到血液中的CMV。且至少有以下症状中的2项:(1)发热≥38 ℃至少2 d;(2)新或加重的不适或疲劳;(3)2次独立检测的白细胞减少或中性粒细胞减少;(4)5%非典型淋巴细胞;(5)血小板减少症;(5)肝移植受者肝转氨酶增加至正常值上限2倍 胃肠道CMV病 存在上消化道和(或)下消化道症状,同时胃肠镜下可见的黏膜病变,以及组织病理学、病毒分离、快速培养、免疫组织化学或DNA杂交技术检测到胃肠道组织有CMV 存在上消化道和(或)下消化道症状和组织中检测到CMV,但胃肠镜下无可见的黏膜病变;仅通过核酸或pp65抗原检测在血液中检测到CMV不足以诊断CMV胃肠道疾病 CMV肺炎 存在肺炎的临床症状和(或)体征,如影像学上的新浸润、缺氧、呼吸急促和(或)呼吸困难,同时病毒分离、快速培养、组织病理学、免疫组织化学或DNA杂交技术检测到肺组织中有CMV 肺炎的临床症状和(或)体征,如影像学上的新浸润、缺氧、呼吸急促和(或)呼吸困难,及通过肺泡灌洗液的病毒分离和快速培养检测到CMV,或肺泡灌洗液中的CMV DNA定量阳性 CMV肝炎 肝功能检查异常[胆红素和(或)肝酶升高],同时组织病理学、免疫组织化学、病毒分离、快速培养或DNA杂交技术在肝组织中检测到CMV,排除其他原因导致的肝炎 未定义 CMV视网
膜炎经眼科医师诊断有典型的CMV视网膜炎体征;如果表现不典型或没有经验丰富的眼科医师参与,则应通过QNAT在玻璃体液中检测到CMV来支持诊断 未定义 CMV脑炎 神经系统症状,同时病毒分离、快速培养、免疫组织化学、原位杂交或QNAT神经系统组织中有CMV 神经系统症状及脑脊液中CMV检测阳性,没有可见的血液污染(“血液抽吸”),同时合并异常的影像学结果 难治性CMV感染 经适当剂量的抗病毒治疗至少2周后,全血或血浆中病毒载量持续增加(即第2周之后检测的病毒载量峰值相比于第1周增加>1 log10) 经适当剂量的抗病毒治疗至少2周后,病毒载量持续存在(即随后第2周之后检测的病毒载量峰值和第1周相同或更高,但增加<1 log10) 难治性CMV病 经适当剂量的抗病毒治疗至少2周后,临床体征和症状恶化或进展 经适当剂量的抗病毒治疗至少2周后,临床体征和症状没有改善 耐药CMV
感染由于CMV基因突变导致其对1种及以上抗病毒药物失去敏感性 未定义 表 3 不同肾功能状态下缬更昔洛韦剂量调整
Table 3. Dose adjustment of valganciclovir under different renal function states
CrCl(mL/min) 诱导剂量 维持剂量/预防剂量 ≥60 900 mg每日2次 900 mg每日1次 40~59 450 mg每日2次 450 mg每日1次 25~39 450 mg每日 1次 450 mg每2日1次 10~24 450 mg每2日1次 450 mg每周2次 <10 不推荐 不推荐 -
[1] KOTTON CN. CMV: prevention, diagnosis and therapy[J]. Am J Transplant, 2013, 13(Suppl 3): 24-40. DOI: 10.1111/ajt.12006. [2] NATORI Y, ALGHAMDI A, TAZARI M, et al. Use of viral load as a surrogate marker in clinical studies of cytomegalovirus in solid organ transplantation: a systematic review and meta-analysis[J]. Clin Infect Dis, 2018, 66(4): 617-631. DOI: 10.1093/cid/cix793. [3] MEESING A, RAZONABLE RR. New developments in the management of cytomegalovirus infection after transplantation[J]. Drugs, 2018, 78(11): 1085-1103. DOI: 10.1007/s40265-018-0943-1. [4] KOTTON CN, KUMAR D, CALIENDO AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation[J]. Transplantation, 2010, 89(7): 779-795. DOI: 10.1097/TP.0b013e3181cee42f. [5] RAZONABLE RR, HUMAR A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice[J]. Clin Transplant, 2019, 33(9): e13512. DOI: 10.1111/ctr.13512. [6] 中华医学会器官移植学分会, 中国医师协会器官移植医师分会. 中国实体器官移植受者巨细胞病毒感染诊疗指南(2016版)[J]. 中华器官移植杂志, 2016, 37(9): 561-565. DOI: 10.3760/cma.j.issn.0254-1785.2016.09.010.Branch of Organ Transplantation of Chinese Medical Association, Branch of Organ Transplant Physician of Chinese Medical Doctor Association. Chinese guidelines for the diagnosis and treatment of cytomegalovirus infection in solid organ transplant recipients (2016 edition)[J]. Chin J Organ Transplant, 2016, 37(9): 561-565. DOI: 10.3760/cma.j.issn.0254-1785.2016.09.010. [7] 中华医学会器官移植学分会. 器官移植受者巨细胞病毒感染临床诊疗规范(2019版)[J]. 器官移植, 2019, 10(2): 142-148. DOI: 10.3969/j.issn.1674-7445.2019.02.005.Branch of Organ Transplantation of Chinese Medical Association. Clinical guidelines for the diagnosis and treatment of cytomegalovirus infection in organ transplant recipients (2019 edition)[J]. Organ Transplant, 2019, 10(2): 142-148. DOI: 10.3969/j.issn.1674-7445.2019.02.005. [8] 陈耀龙, 杨克虎, 王小钦, 等. 中国制订/修订临床诊疗指南的指导原则(2022版)[J]. 中华医学杂志, 2022, 102(10): 697-703. DOI: 10.3760/cma.j.cn112137-20211228-02911.CHEN YL, YANG KH, WANG XQ, et al. Guidelines for the development/revision of clinical guidelines in China (2022 edition)[J]. Natl Med J China, 2022, 102(10): 697-703. DOI: 10.3760/cma.j.cn112137-20211228-02911. [9] Explanation of the 2011 OCEBM Levels of Evidence[EB/OL]. [2023-12-31].https://www.cebm.ox.ac.uk/resources/levels-of-evidence/explanation-of-the-2011-ocebm-levels-of-evidence. [10] LJUNGMAN P, BOECKH M, HIRSCH HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials[J]. Clin Infect Dis, 2017, 64(1): 87-91. DOI: 10.1093/cid/ciw668. [11] CHEMALY RF, CHOU S, EINSELE H, et al. Definitions of resistant and refractory cytomegalovirus infection and disease in transplant recipients for use in clinical trials[J]. Clin Infect Dis, 2019, 68(8): 1420-1426. DOI: 10.1093/cid/ciy696. [12] 巨春蓉, 郝建清, 何建行, 等. 移植受者耐药巨细胞病毒感染的诊治[J]. 中华器官移植杂志, 2023, 44(3): 146-151. DOI: 10.3760/cma.j.cn421203-20220928-00243.JU CR, HAO JQ, HE JX, et al. New progress in diagnosis and treatment of drug-resistant cytomegalovirus infection in transplant recipients[J]. Chin J Organ Transplant, 2023, 44(3): 146-151. DOI: 10.3760/cma.j.cn421203-20220928-00243. [13] CANNON MJ, SCHMID DS, HYDE TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection[J]. Rev Med Virol, 2010, 20(4): 202-213. DOI: 10.1002/rmv.655. [14] FANG FQ, FAN QS, YANG ZJ, et al. Incidence of cytomegalovirus infection in Shanghai, China[J]. Clin Vaccine Immunol, 2009, 16(11): 1700-1703. DOI: 10.1128/CVI.00385-08. [15] WEN L, QIU Y, CHENG S, et al. Serologic and viral genome prevalence of HSV, EBV, and HCMV among healthy adults in Wuhan, China[J]. J Med Virol, 2018, 90(3): 571-581. DOI: 10.1002/jmv.24989. [16] LIMAYE AP, BABU TM, BOECKH M. Progress and challenges in the prevention, diagnosis, and management of cytomegalovirus infection in transplantation[J]. Clin Microbiol Rev, 2020, 34(1): e00043-19. DOI: 10.1128/CMR.00043-19. [17] SILVA JUNIOR HT, TOKAT Y, CAI J, et al. Epidemiology, management, and burden of cytomegalovirus in solid organ transplant recipients in selected countries outside of Europe and North America: a systematic review[J]. Transpl Infect Dis, 2023, 25(4): e14070. DOI: 10.1111/tid.14070. [18] JU C, WANG X, XU X, et al Cytomegalovirus seroprevalence, infection, and disease in Chinese thoracic organ transplant recipients: a retrospective cohort study[J]. BMC Infect Dis, 2022, 22(1): 872. DOI: 10.1186/s12879-022-07853-x. [19] FERNÁNDEZ-RUIZ M, GIMÉNEZ E, VINUESA V, et al. Regular monitoring of cytomegalovirus-specific cell-mediated immunity in intermediate-risk kidney transplant recipients: predictive value of the immediate post-transplant assessment[J]. Clin Microbiol Infect, 2019, 25(3): 381. e1-381. e10. DOI: 10.1016/j.cmi.2018.05.010. [20] TANG Y, GUO J, LI J, et al. Risk factors for cytomegalovirus infection and disease after kidney transplantation: a meta-analysis[J]. Transpl Immunol, 2022, 74: 101677. DOI: 10.1016/j.trim.2022.101677. [21] DE WEERD AE, BETJES MGH. ABO-incompatible kidney transplant outcomes: a meta-analysis[J]. Clin J Am Soc Nephrol, 2018, 13(8): 1234-1243. DOI: 10.2215/CJN.00540118. [22] ZHENG X, GONG L, XUE W, et al. Kidney transplant outcomes in HIV-positive patients: a systematic review and meta-analysis[J]. AIDS Res Ther, 2019, 16(1): 37. DOI: 10.1186/s12981-019-0253-z. [23] MULLER E, BOTHA FCJ, BARDAY ZA, et al. Kidney transplantation in HIV-positive patients: current practice and management strategies[J]. Transplantation, 2021, 105(7): 1492-1501. DOI: 10.1097/TP.0000000000003485. [24] GARDINER BJ, NIERENBERG NE, CHOW JK, et al. Absolute lymphocyte count: a predictor of recurrent cytomegalovirus disease in solid organ transplant recipients[J]. Clin Infect Dis, 2018, 67(9): 1395-1402. DOI: 10.1093/cid/ciy295. [25] MEESING A, ABRAHAM RS, RAZONABLE RR. Clinical correlation of cytomegalovirus infection with CMV-specific CD8+ T-cell immune competence score and lymphocyte subsets in solid organ transplant recipients[J]. Transplantation, 2019, 103(4): 832-838. DOI: 10.1097/TP.0000000000002396. [26] RAZONABLE RR, RIVERO A, RODRIGUEZ A, et al. Allograft rejection predicts the occurrence of late-onset cytomegalovirus (CMV) disease among CMV-mismatched solid organ transplant patients receiving prophylaxis with oral ganciclovir[J]. J Infect Dis, 2001, 184(11): 1461-1464. DOI: 10.1086/324516. [27] RAZONABLE RR, HAYDEN RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation[J]. Clin Microbiol Rev, 2013, 26(4): 703-727. DOI: 10.1128/CMR.00015-13. [28] LAZZAROTTO T, CHIEREGHIN A, PIRALLA A, et al. Kinetics of cytomegalovirus and Epstein-Barr virus DNA in whole blood and plasma of kidney transplant recipients: implications on management strategies[J]. PLoS One, 2020, 15(8): e0238062. DOI: 10.1371/journal.pone.0238062. [29] LEE H, OH EJ. Laboratory diagnostic testing for cytomegalovirus infection in solid organ transplant patients[J]. Korean J Transplant, 2022, 36(1): 15-28. DOI: 10.4285/kjt.22.0001. [30] HIRSCH HH, LAUTENSCHLAGER I, PINSKY BA, et al. An international multicenter performance analysis of cytomegalovirus load tests[J]. Clin Infect Dis, 2013, 56(3): 367-373. DOI: 10.1093/cid/cis900. [31] PRAKASH K, CHANDORKAR A, SAHARIA KK. Utility of CMV-specific immune monitoring for the management of CMV in solid organ transplant recipients: a clinical update[J]. Diagnostics (Basel), 2021, 11(5): 875. DOI: 10.3390/diagnostics11050875. [32] KUMAR D, CHIN-HONG P, KAYLER L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients[J]. Am J Transplant, 2019, 19(9): 2505-2516. DOI: 10.1111/ajt.15315. [33] WESTALL GP, CRISTIANO Y, LEVVEY BJ, et al. A randomized study of quantiferon CMV-directed versus fixed-duration valganciclovir prophylaxis to reduce late CMV after lung transplantation[J]. Transplantation, 2019, 103(5): 1005-1013. DOI: 10.1097/TP.0000000000002454. [34] RUAN Y, GUO W, LIANG S, et al. Diagnostic performance of cytomegalovirus (CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in kidney transplantation: a PRISMA-compliant article[J]. Medicine (Baltimore), 2019, 98(16): e15228. DOI: 10.1097/MD.0000000000015228. [35] DIOVERTI MV, LAHR B, RAZONABLE RR. Treatment of cytomegalovirus infection and disease pre- and post-quantitative nucleic acid test standardization: does use of a more sensitive assay lead to longer treatment duration?[J]. Clin Transplant, 2016, 30(2): 154-160. DOI: 10.1111/ctr.12671. [36] LISBOA LF, KUMAR D, WILSON LE, et al. Clinical utility of cytomegalovirus cell-mediated immunity in transplant recipients with cytomegalovirus viremia[J]. Transplantation, 2012, 93(2): 195-200. DOI: 10.1097/TP.0b013e31823c1cd4. [37] KOTTON CN, KUMAR D, CALIENDO AM, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation[J]. Transplantation, 2013, 96(4): 333-360. DOI: 10.1097/TP.0b013e31829df29d. [38] 张江伟, 燕航, 薛武军, 等. 基于宏基因组二代测序技术检测肾移植术后肺部感染的病原学研究[J]. 中华器官移植杂志, 2021, 42(5): 260-264. DOI: 10.3760/cma.j.cn421203-20210224-00069.ZHANG JW, YAN H, XUE WJ, et al. Pathogeny research of pulmonary infection after renal transplantation based onmetagenomics nextgeneration sequencing[J]. Chin J Organ Transplant, 2021, 42(5): 260-264. DOI: 10.3760/cma.j.cn421203-20210224-00069. [39] LÓPEZ-ALADID R, GUIU A, MOSQUERA MM, et al. Improvement in detecting cytomegalovirus drug resistance mutations in solid organ transplant recipients with suspected resistance using next generation sequencing[J]. PLoS One, 2019, 14(7): e0219701. DOI: 10.1371/journal.pone.0219701. [40] SAM SS, ROGERS R, GILLANI FS, et al. Evaluation of a next-generation sequencing metagenomics assay to detect and quantify DNA viruses in plasma from transplant recipients[J]. J Mol Diagn, 2021, 23(6): 719-731. DOI: 10.1016/j.jmoldx.2021.02.008. [41] BURTON CE, SESTER M, ROBINSON JL, et al. Assigning cytomegalovirus status in children awaiting organ transplant: viral shedding, CMV-specific T cells, and CD27−CD28−CD4+T cells[J]. J Infect Dis, 2018, 218(8): 1205-1209. DOI: 10.1093/infdis/jiy309. [42] BURTON CE, DRAGAN T, MABILANGAN CA, et al. Assignment of cytomegalovirus infection status in infants awaiting solid organ transplant: viral detection methods as adjuncts to serology[J]. Pediatr Transplant, 2018, 22(5): e13229. DOI: 10.1111/petr.13229. [43] PAYA C, HUMAR A, DOMINGUEZ E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients[J]. Am J Transplant, 2004, 4(4): 611-620. DOI: 10.1111/j.1600-6143.2004.00382.x. [44] 王垒, 彭贵主, 叶啟发. 肾移植术后抢先治疗和普遍预防对预防巨细胞病毒感染的Meta分析[J]. 中华泌尿外科杂志, 2018, 39(4): 294-299. DOI: 10.3760/cma.j.issn.1000-6702.2018.04.013.WANG L, PENG GS, YE QF. Clinical values of preemptive therapy versus universal prophylaxis in the prevention of cytomegalovirus infection post kidney transplantation: a systematic review and meta-analysis[J]. Chin J Urol, 2018, 39(4): 294-299. DOI: 10.3760/cma.j.issn.1000-6702.2018.04.013. [45] BALFOUR HH JR, CHACE BA, STAPLETON JT, et al. A randomized, placebo-controlled trial of oral acyclovir for the prevention of cytomegalovirus disease in recipients of renal allografts[J]. N Engl J Med, 1989, 320(21): 1381-1387. DOI: 10.1056/NEJM198905253202105. [46] HEDDLE NM, BOECKH M, GROSSMAN B, et al. AABB committee report: reducing transfusion-transmitted cytomegalovirus infections[J]. Transfusion, 2016, 56(6 Pt 2): 1581-1587. DOI: 10.1111/trf.13503. [47] RAVAL AD, KISTLER KD, TANG Y, et al. Burden of neutropenia and leukopenia among adult kidney transplant recipients: a systematic literature review of observational studies[J]. Transpl Infect Dis, 2023, 25(1): e14000. DOI: 10.1111/tid.14000. [48] 刘凡, 李杨, 丁晨光, 等. 低剂量缬更昔洛韦预防肾移植术后巨细胞病毒感染的疗效观察[J]. 中华器官移植杂志, 2020, 41(4): 227-231. DOI: 10.3760/cma.j.cn421203-20191022-00383.LIU F, LI Y, DING CG, et al. Prophylaxis for cytomegalovirus with low-dose valganciclovir after renal transplantation[J]. Chin J Organ Transplant, 2020, 41(4): 227-231. DOI: 10.3760/cma.j.cn421203-20191022-00383. [49] LOWANCE D, NEUMAYER HH, LEGENDRE CM, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group[J]. N Engl J Med, 1999, 340(19): 1462-1470. DOI: 10.1056/NEJM199905133401903. [50] LIMAYE AP, BUDDE K, HUMAR A, et al. Letermovir vs valganciclovir for prophylaxis of cytomegalovirus in high-risk kidney transplant recipients: a randomized clinical trial[J]. JAMA, 2023, 330(1): 33-42. DOI: 10.1001/jama.2023.9106. [51] BARTEN MJ, BALDANTI F, STAUS A, et al. Effectiveness of prophylactic human cytomegalovirus hyperimmunoglobulin in preventing cytomegalovirus infection following transplantation: a systematic review and meta-analysis[J]. Life (Basel), 2022, 12(3): 361. DOI: 10.3390/life12030361. [52] HODSON EM, JONES CA, STRIPPOLI GF, et al. Immunoglobulins, vaccines or interferon for preventing cytomegalovirus disease in solid organ transplant recipients[J]. Cochrane Database Syst Rev, 2007(2): CD005129. DOI: 10.1002/14651858.CD005129.pub2. [53] SNYDMAN DR, FALAGAS ME, AVERY R, et al. Use of combination cytomegalovirus immune globulin plus ganciclovir for prophylaxis in CMV-seronegative liver transplant recipients of a CMV-seropositive donor organ: a multicenter, open-label study[J]. Transplant Proc, 2001, 33(4): 2571-2575. DOI: 10.1016/s0041-1345(01)02101-7. [54] HUMAR A, LEBRANCHU Y, VINCENTI F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients[J]. Am J Transplant, 2010, 10(5): 1228-1237. DOI: 10.1111/j.1600-6143.2010.03074.x. [55] RAZONABLE RR, BLUMBERG EA. It's not too late: a proposal to standardize the terminology of "late-onset" cytomegalovirus infection and disease in solid organ transplant recipients[J]. Transpl Infect Dis, 2015, 17(6): 779-784. DOI: 10.1111/tid.12447. [56] LA ROSA C, LIMAYE AP, KRISHNAN A, et al. Primary response against cytomegalovirus during antiviral prophylaxis with valganciclovir, in solid organ transplant recipients[J]. Transpl Int, 2011, 24(9): 920-931. DOI: 10.1111/j.1432-2277.2011.01285.x. [57] VAN DER BEEK MT, BERGER SP, VOSSEN AC, et al. Preemptive versus sequential prophylactic-preemptive treatment regimens for cytomegalovirus in renal transplantation: comparison of treatment failure and antiviral resistance[J]. Transplantation, 2010, 89(3): 320-326. DOI: 10.1097/TP.0b013e3181bc0301. [58] WIITA AP, ROUBINIAN N, KHAN Y, et al. Cytomegalovirus disease and infection in lung transplant recipients in the setting of planned indefinite valganciclovir prophylaxis[J]. Transpl Infect Dis, 2012, 14(3): 248-258. DOI: 10.1111/j.1399-3062.2012.00723.x. [59] MANUEL O, HUSAIN S, KUMAR D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study[J]. Clin Infect Dis, 2013, 56(6): 817-824. DOI: 10.1093/cid/cis993. [60] RUENROENGBUN N, NUMTHAVAJ P, SAPANKAEW T, et al. Efficacy and safety of conventional antiviral agents in preventive strategies for cytomegalovirus infection after kidney transplantation: a systematic review and network meta-analysis[J]. Transpl Int, 2021, 34(12): 2720-2734. DOI: 10.1111/tri.14122. [61] WITZKE O, HAUSER IA, BARTELS M, et al. Valganciclovir prophylaxis versus preemptive therapy in cytomegalovirus-positive renal allograft recipients: 1-year results of a randomized clinical trial[J]. Transplantation, 2012, 93(1): 61-68. DOI: 10.1097/TP.0b013e318238dab3. [62] MATTES FM, HAINSWORTH EG, HASSAN-WALKER AF, et al. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir[J]. J Infect Dis, 2005, 191(1): 89-92. DOI: 10.1086/425905. [63] SAGEDAL S, NORDAL KP, HARTMANN A, et al. Pre-emptive therapy of CMVpp65 antigen positive renal transplant recipients with oral ganciclovir: a randomized, comparative study[J]. Nephrol Dial Transplant, 2003, 18(9): 1899-1908. DOI: 10.1093/ndt/gfg302. [64] REUSSER P, EINSELE H, LEE J, et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation[J]. Blood, 2002, 99(4): 1159-1164. DOI: 10.1182/blood.v99.4.1159. [65] CHAKRABARTI S, COLLINGHAM KE, OSMAN H, et al. Cidofovir as primary pre-emptive therapy for post-transplant cytomegalovirus infections[J]. Bone Marrow Transplant, 2001, 28(9): 879-881. DOI: 10.1038/sj.bmt.1703251. [66] LEE JH, LEE H, LEE SW, et al. Efficacy and safety according to the dose of valganciclovir for cytomegalovirus prophylaxis in transplantation: network meta-analysis using recent data[J]. Transplant Proc, 2021, 53(6): 1945-1950. DOI: 10.1016/j.transproceed.2021.05.006. [67] 孙熙木, 王华光, 刘丽宏. 器官移植术后巨细胞病毒感染的药物治疗现状[J]. 中国临床药理学杂志, 2021, 37(15): 2088-2091. DOI: 10.13699/j.cnki.1001-6821.2021.15.036.SUN XM, WANG HG, LIU LH. Research status of drug therapy on cytomegalovirus infection after solid organ transplantation[J]. Chin J Clin Pharm, 2021, 37(15): 2088-2091. DOI: 10.13699/j.cnki.1001-6821.2021.15.036. [68] 周迷, 朱玉莲, 杨勇. 新型抗病毒药物防治移植患者巨细胞病毒感染的研究进展[J]. 医药导报, 2022, 41(4): 458-461. DOI: 10.3870/j.issn.1004-0781.2022.04.006.ZHOU M, ZHU YL, YANG Y. Research Progress of novel antiviral drugs in the treatment and prevention of cytomegalovirus infection in transplant patients[J]. Herald Med, 2022, 41(4): 458-461. DOI: 10.3870/j.issn.1004-0781.2022.04.006. [69] AVERY RK, ALAIN S, ALEXANDER BD, et al. Maribavir for refractory cytomegalovirus infections with or without resistance post-transplant: results from a phase 3 randomized clinical trial[J]. Clin Infect Dis, 2022, 75(4): 690-701. DOI: 10.1093/cid/ciab988. [70] CHACKO B, JOHN GT. Leflunomide for cytomegalovirus: bench to bedside[J]. Transpl Infect Dis, 2012, 14(2): 111-120. DOI: 10.1111/j.1399-3062.2011.00682.x. [71] SANTHANAKRISHNAN K, YONAN N, IYER K, et al. Management of ganciclovir resistance cytomegalovirus infection with CMV hyperimmune globulin and leflunomide in seven cardiothoracic transplant recipients and literature review[J]. Transpl Infect Dis, 2022, 24(1): e13733. DOI: 10.1111/tid.13733. [72] 蔡雪君, 关文达, 马钦海, 等. 青蒿素及其衍生物的抗病毒作用机制研究进展[J]. 现代药物与临床, 2022, 37(3): 653-658. DOI: 10.7501/j.issn.1674-5515.2022.03.037.CAI XJ, GUAN WD, MA QH, et al. Research progress on antiviral mechanism of artemisinin and its derivatives[J]. Drug Clin, 2022, 37(3): 653-658. DOI: 10.7501/j.issn.1674-5515.2022.03.037. [73] ASBERG A, HUMAR A, ROLLAG H, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients[J]. Am J Transplant, 2007, 7(9): 2106-2113. DOI: 10.1111/j.1600-6143.2007.01910.x. [74] HODSON EM, LADHANI M, WEBSTER AC, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients[J]. Cochrane Database Syst Rev, 2013(2): CD003774. DOI: 10.1002/14651858.CD003774.pub4. [75] YOUNG PG, RUBIN J, ANGARONE M, et al. Ganciclovir-resistant cytomegalovirus infection in solid organ transplant recipients: a single-center retrospective cohort study[J]. Transpl Infect Dis, 2016, 18(3): 390-395. DOI: 10.1111/tid.12537. [76] SCHULZ U, SOLIDORO P, MÜLLER V, et al. CMV immunoglobulins for the treatment of CMV infections in thoracic transplant recipients[J]. Transplantation, 2016, 100(Suppl 3): S5-S10. DOI: 10.1097/TP.0000000000001097. [77] CALDÉS A, GIL-VERNET S, ARMENDARIZ Y, et al. Sequential treatment of cytomegalovirus infection or disease with a short course of intravenous ganciclovir followed by oral valganciclovir: efficacy, safety, and pharmacokinetics[J]. Transpl Infect Dis, 2010, 12(3): 204-212. DOI: 10.1111/j.1399-3062.2009.00481.x. [78] OUELLETTE CP. Adoptive immunotherapy for prophylaxis and treatment of cytomegalovirus infection[J]. Viruses, 2022, 14(11): 2370. DOI: 10.3390/v14112370. [79] TAPAN U, KUTLUGUN AA, ARICI M, et al. Postural epigastric pain: a challenging symptom for cytomegalovirus (CMV) gastritis[J]. Ren Fail, 2012, 34(2): 235-236. DOI: 10.3109/0886022X.2011.646883. [80] YERUSHALMY-FELER A, PADLIPSKY J, COHEN S. Diagnosis and management of CMV colitis[J]. Curr Infect Dis Rep, 2019, 21(2): 5. DOI: 10.1007/s11908-019-0664-y. [81] YEH PJ, CHIU CT, LAI MW, et al. Clinical manifestations, risk factors, and prognostic factors of cytomegalovirus enteritis[J]. Gut Pathog, 2021, 13(1): 53. DOI: 10.1186/s13099-021-00450-4. [82] ABBAS A, ZIMMER AJ, FLORESCU D. Viral enteritis in solid-organ transplantation[J]. Viruses, 2021, 13(10): 2019. DOI: 10.3390/v13102019. [83] FISHER AT, BESSOFF KE, NICHOLAS V, et al. Fatal case of perforated cytomegalovirus colitis: case report and systematic review[J]. Surg Infect (Larchmt), 2022, 23(2): 127-134. DOI: 10.1089/sur.2021.173. [84] DURAND CM, MARR KA, ARNOLD CA, et al. Detection of cytomegalovirus DNA in plasma as an adjunct diagnostic for gastrointestinal tract disease in kidney and liver transplant recipients[J]. Clin Infect Dis, 2013, 57(11): 1550-1559. DOI: 10.1093/cid/cit521. [85] 巨春蓉, 韦兵, 练巧燕, 等. 实体器官移植术后巨细胞病毒肺炎的防治策略——ATS巨细胞病毒肺炎的诊治指南解读[J]. 器官移植, 2019, 10(1): 88-90. DOI: 10.3969/j.issn.1674-7445.2019.01.014.JU CR, WEI B, LIAN QY, et al. Prevention and treatment strategy of cytomegalovirus pneumonia after solid organ transplantation: interpretation of the diagnosis and treatment of ATS cytomegalovirus pneumonia[J]. Organ Transplant, 2019, 10(1): 88-90. DOI: 10.3969/j.issn.1674-7445.2019.01.014. [86] CONTRERAS G, HO D. Human cytomegalovirus: a survey of end-organ diseases and diagnostic challenges in solid organ transplant recipients[J]. Curr Opin Organ Transplant, 2022, 27(4): 243-249. DOI: 10.1097/MOT.0000000000000992. [87] SEEHOFER D, RAYES N, TULLIUS SG, et al. CMV hepatitis after liver transplantation: incidence, clinical course, and long-term follow-up[J]. Liver Transpl, 2002, 8(12): 1138-1146. DOI: 10.1053/jlts.2002.36732. [88] DA CUNHA T, WU GY. Cytomegalovirus hepatitis in immunocompetent and immunocompromised hosts[J]. J Clin Transl Hepatol, 2021, 9(1): 106-115. DOI: 10.14218/JCTH.2020.00088. [89] SON G, LEE JY, KIM JG, et al. Clinical features of cytomegalovirus retinitis after solid organ transplantation versus hematopoietic stem cell transplantation[J]. Graefes Arch Clin Exp Ophthalmol, 2021, 259(3): 585-591. DOI: 10.1007/s00417-020-04871-w. [90] Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV[EB/OL]. [2023-12-31].https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-oi/guidelines-adult-adolescent-oi.pdf. [91] ROCHA MARUSSI VH, PEDROSO JL, FREITAS LF, et al. Teaching neuroimages: cytomegalovirus infection mimicking a brain tumor in a kidney transplant recipient[J]. Neurology, 2016, 87(23): e281-e282. DOI: 10.1212/WNL.0000000000003406. [92] HUMAR A, KUMAR D, BOIVIN G, et al. Cytomegalovirus (CMV) virus load kinetics to predict recurrent disease in solid-organ transplant patients with CMV disease[J]. J Infect Dis, 2002, 186(6): 829-833. DOI: 10.1086/342601. [93] SULLIVAN T, BRODGINSKI A, PATEL G, et al. The role of secondary cytomegalovirus prophylaxis for kidney and liver transplant recipients[J]. Transplantation, 2015, 99(4): 855-859. DOI: 10.1097/TP.0000000000000386. [94] GARDINER BJ, CHOW JK, PRICE LL, et al. Role of secondary prophylaxis with valganciclovir in the prevention of recurrent cytomegalovirus disease in solid organ transplant recipients[J]. Clin Infect Dis, 2017, 65(12): 2000-2007. DOI: 10.1093/cid/cix696. [95] ÅSBERG A, HUMAR A, ROLLAG H, et al. Lessons learned from a randomized study of oral valganciclovir versus parenteral ganciclovir treatment of cytomegalovirus disease in solid organ transplant recipients: the VICTOR trial[J]. Clin Infect Dis, 2016, 62(9): 1154-1160. DOI: 10.1093/cid/ciw084. [96] HOSSEINI-MOGHADDAM SM, ROTSTEIN C, HUSAIN S. Effects of the intensity of immunosuppressive therapy on outcome of treatment for CMV disease in organ transplant recipients[J]. Am J Transplant, 2011, 11(2): 407. DOI: 10.1111/j.1600-6143.2010.03355.x. [97] YOSHIMURA N, USHIGOME H, AKIOKA K, et al. The beneficial effect of high-dose mizoribine combined with cyclosporine, basiliximab, and corticosteroids on CMV infection in renal transplant recipients[J]. Clin Exp Nephrol, 2013, 17(1): 127-133. DOI: 10.1007/s10157-012-0669-4. [98] KURAMOTO T, DAIKOKU T, YOSHIDA Y, et al. Novel anticytomegalovirus activity of immunosuppressant mizoribine and its synergism with ganciclovir[J]. J Pharmacol Exp Ther, 2010, 333(3): 816-821. DOI: 10.1124/jpet.109.160630. [99] CHOU S, ERCOLANI RJ, VANARSDALL AL. Differentiated levels of ganciclovir resistance conferred by mutations at codons 591 to 603 of the cytomegalovirus UL97 kinase gene[J]. J Clin Microbiol, 2017, 55(7): 2098-2104. DOI: 10.1128/JCM.00391-17. [100] LIMAYE AP, RAGHU G, KOELLE DM, et al. High incidence of ganciclovir-resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy[J]. J Infect Dis, 2002, 185(1): 20-27. DOI: 10.1086/338143. [101] MYHRE HA, HAUG DORENBERG D, KRISTIANSEN KI, et al. Incidence and outcomes of ganciclovir-resistant cytomegalovirus infections in 1244 kidney transplant recipients[J]. Transplantation, 2011, 92(2): 217-223. DOI: 10.1097/TP.0b013e31821fad25. [102] BHORADE SM, LURAIN NS, JORDAN A, et al. Emergence of ganciclovir-resistant cytomegalovirus in lung transplant recipients[J]. J Heart Lung Transplant, 2002, 21(12): 1274-1282. DOI: 10.1016/s1053-2498(02)00463-1. [103] LODDING IP, JØRGENSEN M, BENNEDBÆK M, et al. Development and dynamics of cytomegalovirus UL97 ganciclovir resistance mutations in transplant recipients detected by next-generation sequencing[J]. Open Forum Infect Dis, 2021, 8(10): ofab462. DOI: 10.1093/ofid/ofab462. [104] GRACIA-AHUFINGER I, GUTIÉRREZ-AROCA J, CORDERO E, et al. Use of high-dose ganciclovir for the treatment of cytomegalovirus replication in solid organ transplant patients with ganciclovir resistance-inducing mutations[J]. Transplantation, 2013, 95(8): 1015-1020. DOI: 10.1097/TP.0b013e31828555ac. [105] AVERY RK, ARAV-BOGER R, MARR KA, et al. Outcomes in transplant recipients treated with foscarnet for ganciclovir-resistant or refractory cytomegalovirus infection[J]. Transplantation, 2016, 100(10): e74-e80. DOI: 10.1097/TP.0000000000001418. [106] MEHTA STEINKE SA, ALFARES M, VALSAMAKIS A, et al. Outcomes of transplant recipients treated with cidofovir for resistant or refractory cytomegalovirus infection[J]. Transpl Infect Dis, 2021, 23(3): e13521. DOI: 10.1111/tid.13521. [107] BONATTI H, SIFRI CD, LARCHER C, et al. Use of cidofovir for cytomegalovirus disease refractory to ganciclovir in solid organ recipients[J]. Surg Infect (Larchmt), 2017, 18(2): 128-136. DOI: 10.1089/sur.2015.266. [108] AVERY RK, MOSSAD SB, POGGIO E, et al. Utility of leflunomide in the treatment of complex cytomegalovirus syndromes[J]. Transplantation, 2010, 90(4): 419-426. DOI: 10.1097/TP.0b013e3181e94106. [109] WOLF DG, SHIMONI A, RESNICK IB, et al. Human cytomegalovirus kinetics following institution of artesunate after hematopoietic stem cell transplantation[J]. Antiviral Res, 2011, 90(3): 183-186. DOI: 10.1016/j.antiviral.2011.03.184. [110] NASHAN B. Induction therapy and mTOR inhibition: minimizing calcineurin inhibitor exposure in de novo renal transplant patients[J]. Clin Transplant, 2013, 27(Suppl 25): 16-29. DOI: 10.1111/ctr.12156. [111] PASCUAL J, ROYUELA A, FERNÁNDEZ AM, et al. Role of mTOR inhibitors for the control of viral infection in solid organ transplant recipients[J]. Transpl Infect Dis, 2016, 18(6): 819-831. DOI: 10.1111/tid.12601. [112] HOSSEINI-MOGHADDAM SM, SHOKOOHI M, SINGH G, et al. A multicenter case-control study of the effect of acute rejection and cytomegalovirus infection on pneumocystis pneumonia in solid organ transplant recipients[J]. Clin Infect Dis, 2019, 68(8): 1320-1326. DOI: 10.1093/cid/ciy682. [113] PERMPALUNG N, KITTIPIBUL V, MEKRAKSAKIT P, et al. A comprehensive evaluation of risk factors for pneumocystis jirovecii pneumonia in adult solid organ transplant recipients: a systematic review and meta-analysis[J]. Transplantation, 2021, 105(10): 2291-2306. DOI: 10.1097/TP.0000000000003576. [114] FISHMAN JA, GANS H, AST Infectious Diseases Community of Practice. Pneumocystis jiroveci in solid organ transplantation: guidelines from the American Society of Transplantation Infectious Diseases Community of Practice[J]. Clin Transplant, 2019, 33(9): e13587. DOI: 10.1111/ctr.13587. [115] JEHN U, SCHÜTTE-NÜTGEN K, BAUTZ J, et al. Clinical features of BK-polyomavirus and cytomegalovirus co-infection after kidney transplantation[J]. Sci Rep, 2020, 10(1): 22406. DOI: 10.1038/s41598-020-79799-6. [116] BLAZQUEZ-NAVARRO A, DANG-HEINE C, WITTENBRINK N, et al. BKV, CMV, and EBV interactions and their effect on graft function one year post-renal transplantation: results from a large multi-centre study[J]. EBioMedicine, 2018, 34: 113-121. DOI: 10.1016/j.ebiom.2018.07.017. [117] TOYODA M, PULIYANDA DP, AMET N, et al. Co-infection of polyomavirus-BK and cytomegalovirus in renal transplant recipients[J]. Transplantation, 2005, 80(2): 198-205. DOI: 10.1097/01.tp.0000165110.78397.93. [118] HERRERA S, BERNAL-MAURANDI J, COFAN F, et al. BK virus and cytomegalovirus coinfections in kidney transplantation and their impact on allograft loss[J]. J Clin Med, 2021, 10(17): 3779. DOI: 10.3390/jcm10173779. [119] DUBRAWKA CA, PROGAR KJ, JANUARY SE, et al. Impact of antimetabolite discontinuation following cytomegalovirus or BK polyoma virus infection in kidney transplant recipients[J]. Transpl Infect Dis, 2022, 24(6): e13931. DOI: 10.1111/tid.13931. [120] MAH DY, AZLIN A, GNANASEGARAM HK, et al. Double trouble: concurrent cytomegalovirus and BK polyomavirus infections in a patient who underwent kidney transplantation[J]. Korean J Transplant, 2020, 34(2): 117-120. DOI: 10.4285/kjt.2020.34.2.117. [121] WU CZ, CHEN XQ, WANG ZY, et al. Simultaneous monitoring of CMV and BKV by quantitative PCR in renal transplant recipients[J]. J Virol Methods, 2014, 210: 40-44. DOI: 10.1016/j.jviromet.2014.09.018. [122] KNIGHT RJ, GRAVISS EA, NGUYEN DT, et al. Conversion from tacrolimus-mycophenolate mofetil to tacrolimus-mTOR immunosuppression after kidney-pancreas transplantation reduces the incidence of both BK and CMV viremia[J]. Clin Transplant, 2018, 32(6): e13265. DOI: 10.1111/ctr.13265. [123] YE C, LI J, LIU X, et al. The incidence of cytomegalovirus and BK polyomavirus infections in kidney transplant patients receiving mTOR inhibitors: a systematic review and meta-analysis[J]. Pharmacotherapy, 2023, 43(6): 552-562. DOI: 10.1002/phar.2799. [124] BANKO A, MILJANOVIC D, CIRKOVIC A. Systematic review with meta-analysis of active herpesvirus infections in patients with COVID-19: old players on the new field[J]. Int J Infect Dis, 2023, 130: 108-125. DOI: 10.1016/j.ijid.2023.01.036. [125] NAENDRUP JH, GARCIA BORREGA J, EICHENAUER DA, et al. Reactivation of EBV and CMV in severe COVID-19-epiphenomena or trigger of hyperinflammation in need of treatment? a large case series of critically ill patients[J]. J Intensive Care Med, 2022, 37(9): 1152-1158. DOI: 10.1177/08850666211053990. [126] CHEN L, SHEN L, WU W, et al. Co-infecting pathogens can contribute to inflammatory responses and severe symptoms in COVID-19[J]. J Thorac Dis, 2022, 14(2): 355-370. DOI: 10.21037/jtd-21-1284. [127] JORGENSON MR, DESCOUROUEZ JL, WONG C, et al. Cytomegalovirus antiviral stewardship in the COVID-19 era: increasing complexity of prophylaxis and treatment and potential mitigation strategies[J]. Transpl Infect Dis, 2021, 23(4): e13586. DOI: 10.1111/tid.13586. [128] HELLEMANS R, ABRAMOWICZ D. Cytomegalovirus after kidney transplantation in 2020: moving towards personalized prevention[J]. Nephrol Dial Transplant, 2022, 37(5): 810-816. DOI: 10.1093/ndt/gfaa249. -





 下载:
下载: